Fatty acid composition of soybean/sunflower mix oil, fish oil and butterfat applying the AOCS Ce 1j-07 method with a modified
temperature program
L.
Massona,b,
T.
Alfaroc,
C.
Camiloa,
A.
Carvalhob,
P.
Illescad,
R.
Torrese,
M.
Tavares do Carmob,
J.
Mancini-Filhoe and C.
Bernald,*
aCentro de Investigación y Desarrollo en Grasas y Aceites - CIDGRA, Universidad de Chile, Santiago, Chile
bInstituto de Nutrição Josué de Castro, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil
cInstituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA), San José, Costa Rica
dBromatología y Nutrición, Facultad de Bioquímica y Ciencias Biológicas, Universidad Nacional del Litoral, Santa Fe, Argentina
eFaculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, Brasil
*Corresponding autor: cbernal@fbcb.unl.edu.ar
| |
SUMMARY
Gas-Liquid Chromatography (GLC) methods such as AOAC Fat in foods 966.06 (2005), AOCS Official Methods Ce 1h-05 (2005), Ce
1j-07 (2007), allow for analyzing the fatty acids (FAs) in dietary fats using highly polar liquid phase capillary columns.
However, there are still difficulties in completely separating butiric acid from solvent, FA critical pairs with similar polarity,
conjugated linoleic acid (CLA) isomers, and long chain-polyunsaturated FAs (LC-PUFAs). Therefore, the selection of the temperature
program to be employed is important. This work aimed to improve the AOCS Ce 1j-07 Method for the FA composition of a mixture
of soybean and sunflower oil, fish oil, and butterfat, using a modified temperature program, tested among five laboratories.
It takes more time, but it allows to completely separate butyric acid from the solvent, trans-18:1 from cis-18:1, 20:1 isomers from 18:3 n-3, 22:1 n-9 from 20:4 n-6, 20:5 n-3 from 24:0 and the main CLA isomers, thus permitting FA
quantification in fats and oils for different purposes such as nutritional labeling, quality control and research.
|
| |
RESUMEN
Composición en ácidos grasos de mezcla de aceite de soja y girasol, aceite de pescado y mantequilla por el método AOCS Ce
1j-07 usando un programa de temperatura modificado. Métodos por cromatografía gas-líquido, AOAC 966.06 (2005), AOCS Ce 1h-05 (2005), Ce 1j-07 (2007) permiten determinar ácidos
grasos (AG) en matri-ces grasas usando columnas capilares altamente polares y distintos programas de temperatura. No obstante,
aún existen dificultades para separar ácido butírico del solvente, pares críticos de AG con polaridades similares, isómeros
del ácido linoleico conjugado (CLA), AG de cadena larga poliinsaturados (LC-PUFAs). El objetivo fue mejorar el Método AOCS
Ce 1j-07 aplicándolo a la composición en AG de mezcla de aceite soja/girasol, aceite de pescado, mantequilla, usando un programa
de temperatura modificado, entre cinco laboratorios. El programa de temperatura elegido, si bien emplea más tiempo, permite
separar completamente ácido butírico del solvente, trans-18:1 de cis-18:1, isómeros 20:1 de 18:3 n-3, 22:1 n-9 de 20:4 n-6, 20:5 n-3 de 24:0, los principales isómeros CLA. Esta propuesta permite
cuantificar AG con diferentes propósitos, entre ellos, etiquetado nutricional, control de calidad e investigación.
|
1. INTRODUCTIONTOP
The analysis of fatty acids (FAs) is required for many applications in food science such as nutritional labeling, quality
control, composition databases, traceability for international markets, nutrition and health, medical purposes and research.
The method for analyzing FAs should include the maximum resolution and identification of FAs, covering from short chain to
long chain FAs, saturated (SFAs), monounsaturated (MUFAs), polyunsaturated (PUFAs), long chain-PUFAs (LC-PUFAs), as well as
positional and geometrical FA isomers, naturally or industrially produced. The main procedures for FA analysis in different
matrixes are based on GLC methods (Seppänen-Laakso et al., 2002; Christie et al., 2007; Smith and Hansen, 2008; Mossoba and Kramer, 2009). Complementary techniques such as thin-layer chromatography impregnated with silver nitrate and silver-ion liquid chromatography
(Kramer et al., 1998), Fourier transform infrared spectroscopy (Van de Voort et al., 2008; Mossoba et al., 2009), and mass spectrometry (Ratnayake, 2004; Manzano et al., 2012) are also used.
The identification and quantification of SFAs, MUFAs, PUFAs, LC-PUFAs, trans-FA (TFA) isomers by direct GLC has been improved in recent years using high efficiency silica capillary columns, 100 m coated
with not bonded highly polar stationary liquid phase 100% cyanopropyl polysiloxane, such as SP-2560, CP Sil 88, Rtx-2560 (Mossoba and Kramer, 2009). However, using these columns, certain difficulties still exist to completely separate short chain FAs such as butyric acid
from the peak solvent, some critical pairs with similar polarity, conjugated linoleic acid (CLA) isomers present in ruminant
fats (Mossoba and Kramer, 2009), along with the identification and quantification of LC-PUFAs mainly with different positions and the quantity of double
bonds in fish oils (Kramer et al., 2008). Thus, the selection of GLC parameters such as the temperature program is analytically very important and it mainly depends
on the complexity of the FAs present in the sample and the purpose of the analysis. These issues must be known by the analyst,
and good reviews on these topics are available in the literature (Seppäenen-Laakso et al., 2002; Kramer et al., 2004; Ratnayake et al., 2006; Destaillats et al., 2007; Rozema et al., 2008; Mossoba and Kramer, 2009; Ruiz-Rodriguez et al., 2010). There are also several standardized methods such as: AOAC Fat in foods 966.06 (2005), AOCS Official Method Ce 1h-05 (2005), AOCS Official Method Ce 1j-07 (2007), AOCS Official Method Ce 2b-11 (2011) and AOCS Official Method Ce 2c-11 (2011). They use GLC, considering direct or previous fat extraction, with differences in stationary liquid phases, internal standards,
and procedures to prepare FA methyl esters (FAMEs). When the conditions of direct GLC are not sufficient for a good separation,
combined methodologies must be used (Kramer et al., 2008).
The aim of this study was to improve Method AOCS Ce 1j-07, using a modified temperature program, for identifying and quantifying
a wide spectrum of FAs present in a sample of soybean and sunflower mixed oil, fish oil and butterfat. It takes more time,
but it allows for a complete separation of butyric acid from the solvent, trans-18:1 from cis-18:1, 20:1 isomers from 18:3 n-3, 22:1 n-9 from 20:4 n-6, 20:5 n-3 from 24:0, the main CLA isomers. This proposal permits
FA quantification in fats and oils for different purposes such us nutritional labeling, quality control, and research.
2. MATERIALS AND METHODSTOP
2.1. Standards, chemicals and samplesTOP
Internal standard (IS) Tritridecanoine [13:0-triacylglycerol (TAG)], external standards GLC-463 Reference Standard containing
52 FAME mixture (purity >99%) and trans-mix GLC 481 (purity >99%) were purchased from Nu-Chek (Nu-Chek Prep, Inc., Elysian, MN, USA). Beef-Pork Fat Blend Certified
Reference Material (N° 1061, BCR 163) was purchased from the Joint Research Center, Institute for Reference Materials and
Measurements (IRMM, Geel, Belgium). Linoleic acid methyl esters, cis/trans mix (Catalog n° 47791); FAME mix: C4-C24 unsaturated (Catalog N° 18919) and individual FAMEs from 4:0 to 24:1 chain length
saturated and unsaturated were obtained from Supelco (Bellefonte, PA, USA). GLC-463 standard dissolved in hexane (20 mg·mL−1) was used for calculating the empirical correction factors (ECFs) for each one of its 52 FAMEs and for the fat samples’ FAME
identification, together with other FAME standards. The IS, 13:0-TAG prepared in hexane (5 mg·mL−1) was used for calculating g FAME·100g−1 FAME according to the AOCS Method Ce 1j-07. N-Hexane HPLC grade; sodium chloride p.a.; sodium sulfate anhydrous p.a.; sodium
hydroxide p.a.; methanol p.a. Merck (Hohenbrunn, Germany), BF3 14% in methanol Sigma-Aldrich (St. Louis, MO) were used.
The fat samples were selected according to their different FA compositions and were purchased from local commercial sources:
a) Refined mixture of soybean and sunflower oil (80/20), (Santa Fe, Argentina); b) butterfat (Santa Fe, Argentina); c) fish
oil soft gel capsules (Santiago, Chile).
The fat samples, the same lots of FAME standards and BCR 163 reference material were distributed among the five participating
laboratories from the following institutions: Universidad Nacional del Litoral (UNL), Santa Fe, Argentina; Universidade Federal
do Rio de Janeiro (UFRJ), Río de Janeiro, Brazil; Universidade de São Paulo (USP), São Paulo, Brazil; Universidad de Chile
(UCH), Santiago, Chile and INCIENSA, Ministerio de Salud, San José, Costa Rica. All these materials were maintained at −23
°C until they were analyzed.
2.2. Other materialsTOP
New fused silica capillary columns SPTM-2560 0.25 mm i.d. × 100 m length, coated with 100% cyanopropyl polysiloxane stationary phase, film thickness 0.20 μm, were
used in four laboratories (Supelco, Inc., Bellefonte, PA, USA, Part N° 24056), one laboratory used CP SilTM88 with the same characteristics (Varian, Walnut Creek, CA, USA, Part N° CP7489). Focus Liner with glass wool (Catalog N°21022-211.5,
Restek or equivalent Supelco, Sigma–Aldrich, St. Louis, MO), and capped test tubes Pyrex USA, N° 9826, with teflon liner,
150 mm × 20 mm (Fisher Scientific Corp., USA) were used. The following gases were employed: as carrier gas helium or hydrogen,
as make up gas of nitrogen, hydrogen and air for the flame ionization detector (FID) of chromatography quality.
2.3. Instrumentation and analytical conditionsTOP
The GLC instruments located at the five laboratories from the participating institutions were the following: UNL/Argentina:
Shimadzu GC 2014 with GC Solutions software; UFRJ/Brazil: Agilent 7890 A and EZ Chrom Elite; USP/Brazil: Shimadzu GC 17A and
Class GC 10; UCH/Chile: HP 5890 Serie II and Clarity Chromatography SW Data Apex 2006, Waters; INCIENSA/Costa Rica: Agilent
7890 A with Chemstation Agilent, with FID detector (air to hydrogen ratio, 400:40). The injector and detector temperatures
were maintained at 250 °C, split ratio 1:100, 1 μL of standard or sample, equivalent to 20 μg of total FAMEs were injected
using an autosampler device in each GLC run. The fluxes of hydrogen and helium were 1mL·min−1 and 2 mL·min−1, respectively, the nitrogen flux as make up gas was 25 mL·min−1.
2.4. Correction factorsTOP
The theoretical correction factor (TCFr) relative to the IS (13:0-TAG) was used to correct the FID response for the quantitative
expression of each FAME (g FAME·100 g−1 FAME). The TCFr for short chain FAs and LC-PUFAs did not show a proper quantitative response by FID, with the real possibility
of underestimating short chain FAs or to overestimate LC-PUFAs. Then, each laboratory experimentally determined the empirical
correction factor (ECF) for each one of the 52 FAMEs present in GLC 463, from butyric acid to DHA, using g % and % purity indicated
in the Certificate of Analysis. These calculated ECFs were used for the FAMEs quantification in the Reference Certified Material
BCR 163, and in the commercial samples. Even the Reference Standard GLC 714 is recommended by the AOCS Official Method Ce 1j-07 (2007); Reference Standard GLC 463 has been used for its important comparative advantages: it contains 52 FAMEs with a wide spectrum
of FAs from 4:0 to 24:1, most of them present in the fat matrixes analyzed in this study. The FAMEs are in different percentages,
1%, 2% and 4%, including critical pairs with close polarity, which permits a good separation, clear identification according
to their respective relative retention times to 18:0 in SP-2560 and CP Sil 88 columns and their quantification. Standard mixture
GLC 714 has only 24 FAMEs, and is missing key FAMEs, such as 4:0, 5:0, 6:0, which are fundamental for the butterfat analysis.
2.5. BCR-163 certified reference material, identification and quantificationTOP
In order to check the quantitative performance of the modified temperature program, the seven certified FAMEs were quantified
and compared, including as appropriate their positional and geometric isomers present in the BCR-163 certified reference material
using the calculated ECF and tabulated TCFr. The percentage of recovery for each certified FAME was calculated by each laboratory
considering the analytical value obtained and the certified value declared by the Institute for Reference Materials and Measurements
(Geel, Belgium).
In accordance with the procedure of the AOCS Official Method Ce 1j-07 (2007), 1 mL of the IS TAG 13:0 solution containing 5 mg was added to 0.100 ± 0.001 g of BCR-163 and weighed at least in triplicate.
For identification, the relative retention time of each FAME to 18:0 was used, and compared with those obtained for the FAMEs
present in GLC 463, and literature references.
2.6. Fat samples: quantification, derivatization and identification of fatty acidsTOP
To 0.100 ± 0.001 g of each anhydrous fat samples, soybean and sunflower oil mixture l (80/20), fish oil and butterfat, 1 mL
of the IS solution containing 5 mg of 13:0-TAG was added, followed by the FAME derivatization procedure using BF3 14% in methanol according to International Standards – ISO 5509 (2000), indicated by the AOCS Method Ce 1j-07. The FAMEs
in the fat samples were identified by the GLC procedure using the modified temperature program, by comparison of their relative
retention times calculated to 18:0 with the respective relative retention times of the 52 FAMEs in the GLC-463 standard, other
reference FAME materials mentioned in 2.1 and in the literature. The results for the soybean/sunflower oil mixture were determined
using its respective calculated ECF and tabulated TCFr values. For fish oil and anhydrous butterfat only ECF values were used
for the quantitative procedure. The final results were expressed in g FAME·100 g−1 FAMEs, g FA/100 g FAs and TAG equivalents % (TAGe), according to the AOCS Method Ce 1j-07 (2007).
2.7. Statistical analysisTOP
In the five laboratories, the z score was calculated for each one of the seven certified FAMEs of the reference certified
material BCR 163, using their respective calculated ECF, according to IUPAC (Thompson et al., 2006). ANOVA statistical analysis at 95% confidence was used for testing if there were significant differences among the values
obtained for the certified FAMEs using ECF and TCFr and applying the Statgraphics Plus 5.1 statistical program.
3. RESULTS AND DISCUSSIONTOP
3.1. Temperature program and GLC checking performanceTOP
The AOCS Ce 1j-07 (2007) initial isotherm temperature program, i.e. 180 °C (held for 32 min), icreased at a rate of 20 °C·min−1 to 215 °C (held for 31.25 min), for a total time 50 min was assayed running the GLC 463 standard. Figure 1A shows the best chromatogram obtained. In general, 51 peaks of the 52 FAMEs were detected. The short chain FAMEs, including
butyric acid, eluted very close to the solvent peak, and in some of the five participant laboratories (data not shown) it
could not be separated completely from the solvent peak; 20:5 n-3 emerged together with 24:0, and overlapping of some critical
FA pairs was observed. These analytical difficulties were considered of importance to modify the temperature program, because
butterfat and fish oil were part of the test samples analyzed in this study. Mild temperature conditions at the beginning
of the GLC run, slower rate temperature in the middle zone and speeding the elution of the LC-PUFAs at the end of the run
were tested. After assaying different approaches, the following temperature program was selected and assayed among the five
laboratories: an initial temperature of 100 °C increased at a rate of 3 °C·min−1 to 140 °C, at a rate of 0.5 °C·min−1 to 170 °C, increased ar a rate of 4 °C per min−1 to 220 °C, and maintained for 30 min. This modified temperature program optimized the separation of the 52 FAMEs present
in GLC 463, and took 110 min, as can be clearly observed in Figure 1B.
|
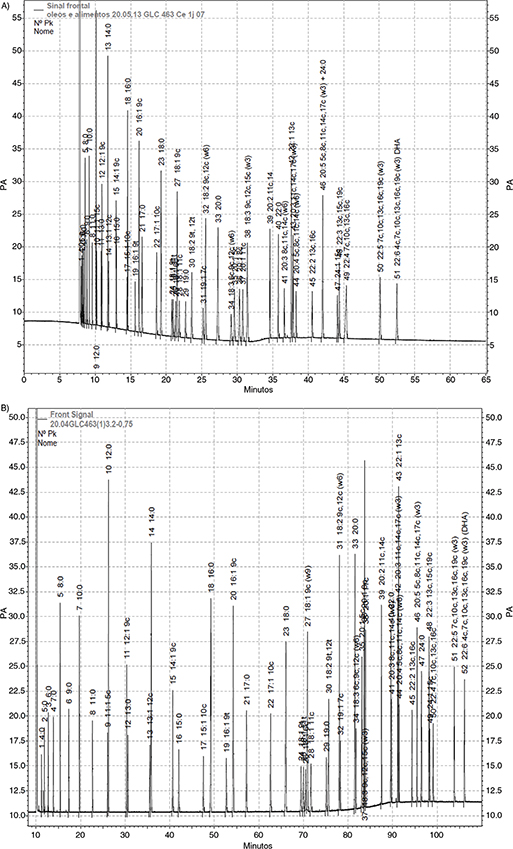 Figure 1. A. Representative chromatogram of GLC 463 Certified Reference Standard using the AOCS Official Method Ce 1j-07. B. Representative
chromatogram of GLC 463 Certified Reference Standard using the proposed temperature program (see paragraph 3.1 for operating
conditions). Figure 1. A. Representative chromatogram of GLC 463 Certified Reference Standard using the AOCS Official Method Ce 1j-07. B. Representative
chromatogram of GLC 463 Certified Reference Standard using the proposed temperature program (see paragraph 3.1 for operating
conditions).
|
|
Even considering this longer chromatographic run, the positive results obtained with this modified temperature program permitted
a clear resolution for very short chain FAs. Butyric acid became quite separated from the peak of solvent, and the overlapping
of critical pairs was properly resolved, such as 14:1 with 15:0, 15:1 with 16:0, trans and cis 18:1 isomers; 18:2 n-6 isomers; 20:1 isomers from 18:3 n-3; 20:0 from 18:3 n-3; 22:0 from 20:3 n-3; 22:1 n-9 from 20:4 n-6,
20:5 n-3 from 24:0. In addition, odd and even carbon chain-FAs, were well separated. With respect to the GLC checking performance,
the partition obtained with this modified GLC temperature program was complete between the base line of 9c-18:1 and 11c-18:1, and the resolution was close to 1.0 between 11c-20:1 and 9c,12c,15c-18:3. Therefore, this good resolution of the 52 FAMEs throughout the chromatographic run allows for the use of this modified
temperature program for FA identification and quantification in a wide variety of foods, including dairy products, animal
fats, vegetable and fish oils, with a complex FA composition, maintaining the general procedure described in the AOCS Method
Ce 1j-07 (2007).
The analytical performance of the capillary columns SPTM-2560 or CP SilTM 88 used in this study yielded similar analytical results with hydrogen or helium as carrier gas.
3.2. Correction factorsTOP
The values obtained for the ECF determined experimentally by the five laboratories for each one of the 52 FAMEs in GLC 463
are presented in Table 1. They were compared with the respective tabulated TCFr. The difference obtained between both values was expressed as a % according
to the AOCS Official Method Ce 1j-07 (2007) formula. Important differences between ECF and TCFr values were obtained in the
extremes for short chain FAs and LC-PUFAs, confirming that for fats containing these types of FA, determined ECF values must
be used (Mossoba and Kramer, 2009). In addition, the AOCS Method Ce 1j-07 (2007) does not provide TCFr for 20:3, 20:4, 20:5, 22:5 and 22:6 FAMEs, and therefore
calculated ECF must be used. The ECF values for FAMEs of 8–12 carbon atoms showed a more homogeneous tendency. For medium
chain FAs, mainly saturated with 14–18 carbons, the difference between both values was lower than 3%, confirming that the
GLC systems of the five laboratories worked properly. The ECF data corresponded to the mean value obtained from triplicates.
Table 1. Comparison of ECF vs. TCFr values for the 52 FAMEs of the GLC-463 Reference Material
| FAME |
TCFr |
Lab 1 |
Lab 2 |
Lab 3 |
Lab 4 |
Lab 5 |
| ECF |
Δ (%)
|
ECF |
Δ (%)
|
ECF |
Δ (%)
|
ECF |
Δ (%)
|
ECF |
Δ (%)
|
| 4:0 |
1.4534 |
1.6450 |
13.2 |
1.7302 |
19.0 |
2.3807 |
63.8 |
1.9436 |
33.7 |
2.4265 |
67.0 |
| 5:0 |
1.3224 |
1.4550 |
10.1 |
1.4328 |
8.3 |
1.5614 |
18.1 |
1.6213 |
22.6 |
1.8000 |
36.1 |
| 6:0 |
1.2351 |
1.4060 |
13.9 |
1.3053 |
5.7 |
1.3169 |
6.6 |
1.4835 |
20.1 |
1.5540 |
25.8 |
| 7:0 |
1.1727 |
1.3510 |
15.3 |
1.3326 |
13.6 |
1.3621 |
16.1 |
1.4049 |
19.8 |
1.4101 |
20.2 |
| 8:0 |
1.1259 |
1.1740 |
4.3 |
1.1639 |
3.4 |
1.1696 |
3.9 |
1.1988 |
6.5 |
1.1944 |
6.1 |
| 9:0 |
1.0896 |
1.1440 |
5.0 |
1.1177 |
2.6 |
1.1329 |
4.0 |
1.1356 |
4.2 |
0.9661 |
-11.3 |
| 10:0 |
1.0604 |
1.0980 |
3.6 |
1.0894 |
2.7 |
1.0872 |
2.5 |
1.1147 |
5.1 |
1.0908 |
2.9 |
| 11:0 |
1.0366 |
1.1020 |
6.8 |
1.0595 |
2.7 |
1.0636 |
3.1 |
1.1000 |
6.1 |
1.0853 |
5.2 |
| 5c-11:1
|
1.0262 |
1.1220 |
9.4 |
1.1136 |
8.5 |
1.1108 |
8.2 |
1.1204 |
9.2 |
1.1095 |
8.1 |
| 12:0 |
1.0168 |
1.0180 |
0.2 |
1.0444 |
2.7 |
1.0165 |
0.0 |
1.0570 |
3.9 |
1.0240 |
0.7 |
| 9c-12:1
|
1.0072 |
1.0620 |
5.5 |
1.0443 |
3.7 |
1.0432 |
3.6 |
1.0707 |
6.3 |
1.0433 |
3.6 |
| 13:0 |
1.0000 |
1.0000 |
0.0 |
1.0000 |
0.0 |
1.0000 |
0.0 |
1.0000 |
0.0 |
1.0000 |
0.0 |
| 12c-13:1
|
0.9912 |
1.0635 |
7.3 |
1.0205 |
3.0 |
1.0425 |
5.2 |
1.0601 |
6.9 |
1.0339 |
4.3 |
| 14:0 |
0.9856 |
0.9925 |
0.7 |
0.9971 |
1.2 |
0.9814 |
-0.4 |
1.0144 |
2.9 |
0.9915 |
0.6 |
| 9c-14:1
|
0.9774 |
1.0177 |
4.2 |
1.0046 |
2.8 |
1.0130 |
3.6 |
1.0230 |
4.7 |
1.0097 |
3.3 |
| 15:0 |
0.9731 |
0.9982 |
2.6 |
0.9675 |
-0.6 |
0.9881 |
1.5 |
0.9713 |
-0.2 |
0.9783 |
0.5 |
| 10c-15:1
|
0.9655 |
1.0148 |
5.1 |
0.9778 |
1.3 |
1.0290 |
6.6 |
0.9918 |
2.7 |
1.0037 |
4.0 |
| 16:0 |
0.9622 |
0.9654 |
0.4 |
0.9545 |
-0.8 |
0.9667 |
0.5 |
0.9685 |
0.7 |
0.9580 |
-0.4 |
| 9t-16:1
|
0.9550 |
0.9725 |
1.9 |
0.9547 |
0.0 |
1.0072 |
5.5 |
0.9704 |
1.6 |
0.9587 |
0.4 |
| 9c-16:1
|
0.9550 |
0.9795 |
2.6 |
0.9604 |
0.6 |
0.9667 |
1.2 |
0.9713 |
1.7 |
0.9768 |
2.3 |
| 17:0 |
0.9526 |
0.9868 |
3.6 |
0.9462 |
-0.7 |
0.9743 |
2.3 |
0.9849 |
1.0 |
0.9558 |
0.3 |
| 10c-17:1
|
0.9458 |
0.9852 |
4.2 |
0.9568 |
1.2 |
0.9922 |
4.9 |
1.0030 |
6.0 |
0.9767 |
3.3 |
| 18:0 |
0.9440 |
0.9621 |
1.9 |
0.9067 |
-3.9 |
0.9526 |
0.9 |
0.9546 |
1.1 |
0.9390 |
-0.5 |
| 9t-18:1
|
0.9377 |
1.0040 |
7.1 |
0.9441 |
0.7 |
0.9812 |
4.6 |
1.0394 |
10.8 |
0.9669 |
3.1 |
| 11t-18:1
|
0.9377 |
0.9847 |
5.0 |
0.9360 |
-0.2 |
0.9735 |
3.8 |
0.9557 |
1.9 |
0.9364 |
-0.1 |
| 6c-18:1
|
0.9377 |
0.9921 |
5.8 |
0.9949 |
6.1 |
0.9990 |
6.5 |
1.0229 |
9.1 |
0.9544 |
1.8 |
| 9c-18:1
|
0.9377 |
0.9377 |
0.0 |
0.9497 |
1.3 |
0.9419 |
0.4 |
0.9708 |
1.0 |
0.9359 |
-0.2 |
| 11c-18:1
|
0.9377 |
0.9632 |
2.8 |
0.9366 |
-0.1 |
0.9757 |
4.0 |
0.9609 |
1.0 |
0.9384 |
0.1 |
| 9t,12t-18:2
|
0.9313 |
0.9983 |
7.2 |
0.9375 |
0.7 |
0.9969 |
7.0 |
1.037 |
11.6 |
0.9756 |
4.8 |
| 9c,12c-18:2
|
0.9313 |
0.9400 |
1.0 |
0.9372 |
0.6 |
0.9459 |
1.6 |
0.9880 |
6.1 |
0.9390 |
0.8 |
| 6c,9c,12c-18:3
|
0.9249 |
1.0419 |
12.7 |
0.9458 |
2.3 |
0.9576 |
3.5 |
0.9919 |
6.5 |
0.9302 |
0.6 |
| 9c,12c,15c-18:3
|
0.9249 |
0.9259 |
0.1 |
0.9547 |
3.2 |
0.9410 |
1.7 |
0.9879 |
6.8 |
0.9405 |
0.2 |
| 19:0 |
0.9364 |
0.9895 |
5.7 |
0.9074 |
-3.1 |
0.8798 |
-6.0 |
0.9921 |
5.9 |
0.9079 |
-3.0 |
| 7c-19:1
|
0.9303 |
0.9338 |
0.4 |
0.9171 |
-1.4 |
0.9449 |
1.6 |
1.0012 |
7.6 |
0.9390 |
0.8 |
| 20:0 |
0.9295 |
0.9712 |
4.5 |
0.9164 |
-1.4 |
0.9186 |
-1.2 |
0.9953 |
1.1 |
0.9400 |
0.6 |
| 5c-20:1
|
0.9237 |
0.9777 |
5.9 |
0.9535 |
3.2 |
0.9350 |
1.2 |
1.0251 |
10.9 |
0.9312 |
0.2 |
| 8c-20:1
|
0.9237 |
0.9960 |
7.9 |
0.8841 |
-4.3 |
0.9019 |
-2.3 |
1.0201 |
10.4 |
0.9258 |
0.8 |
| 11c-20:1
|
0.9237 |
0.9724 |
5.3 |
0.9271 |
0.4 |
0.9154 |
-0.9 |
1.0209 |
10.5 |
0.9273 |
1.7 |
| 11c,14c-20:2
|
0.9180 |
0.9612 |
4.7 |
0.9250 |
0.8 |
0.9268 |
1.0 |
1.0080 |
9.8 |
0.9241 |
0.4 |
| 8c,11c,14c-20:3
|
– |
0.9278 |
– |
0.9166 |
– |
0.9409 |
– |
1.0141 |
– |
0.9272 |
– |
| 11c,14c,17c-20:3
|
– |
0.9466 |
– |
0.9269 |
|
0.9247 |
|
1.0120 |
|
0.9336 |
|
| 5c,8c,11c,14c-20:4
|
– |
0.8855 |
– |
0.9467 |
– |
0.8793 |
– |
0.9754 |
|
0.9034 |
– |
| 5c,8c,11c,14c,17c-20:5
|
– |
0.9371 |
– |
0.9364 |
– |
0.9478 |
– |
1.0474 |
– |
0.8891 |
– |
| 22:0 |
0.9176 |
1.0016 |
9.8 |
0.8893 |
-2.5 |
0.8941 |
-0.2 |
0.9663 |
5.3 |
0.9096 |
1.6 |
| 13c-22:1
|
0.9124 |
0.9459 |
4.3 |
0.9058 |
-0.2 |
0.8995 |
-0.9 |
1.0426 |
14.2 |
0.9022 |
-0.3 |
| 13c,16c-22:2
|
0.9071 |
0.9624 |
6.1 |
0.8832 |
-2.7 |
0.8980 |
-1.0 |
1.0472 |
16.1 |
0.9238 |
2.8 |
| 13c,16c,19c-22:3
|
0.9019 |
0.9503 |
5.4 |
0.9025 |
0.1 |
0.9213 |
2.0 |
1.0787 |
19.6 |
0.9472 |
6.1 |
| 7c,10c,13c,16c-22:4
|
0.8967 |
0.9112 |
1.7 |
0.9255 |
3.2 |
0.9270 |
3.3 |
1.0510 |
17.2 |
0.9211 |
0.5 |
| 7c,10c,13c,16c,19c-22:5
|
– |
0.9369 |
– |
0.9361 |
– |
0.9613 |
– |
1.1128 |
– |
0.9682 |
– |
| 4c,7c,10c,13c,16c,19c-22:6
|
– |
0.9448 |
– |
0.9538 |
– |
0.9855 |
– |
1.1391 |
– |
0.9954 |
– |
| 24:0 |
0.9076 |
0.9759 |
8.7 |
0.8900 |
-0.9 |
0.8850 |
-1.5 |
1.0579 |
16.5 |
0.9525 |
-1.3 |
| 15c-24:1
|
0.9029 |
0.9073 |
1.6 |
0.8881 |
-0.6 |
0.8930 |
-0.1 |
1.0344 |
14.6 |
0.8977 |
5.0 |
| Abbreviations: TCFr, Theoretical correction factor; ECF, Empirical correction factor; Δ, Difference between TCFr and ECF expressed
as percentage. Values are the mean of triplicates.
|
3.3. BCR-163 certified reference materialTOP
A total of 49 FAMEs were identified and quantified in the reference standard BCR-163; 26 FAMEs corresponded to the seven certified
FAMEs including, as appropriate, their positional and geometrical isomers. The mean ± SD (n = 5) and recovery percentage for
each certified FAME were calculated from the data presented by the five laboratories, reaching a final mean of 92.84 and 92.01
g·100g−1 total FAME using ECF and TCFr with recovery percentages of 97.4% and 96.5%, respectively (Table 2). The results for the seven certified FAMEs 14:0; 16:0; 16:1; 18:0; 18:1; 18:2 and 18:3, were in agreement with the theoretical
approach, because ECF and TCFr values for SFAs, MUFAs and PUFAs between 14 and 18 carbon atoms are close to one (Table 1). Therefore, either correction factor can be used to quantify these FAs in edible vegetable oils, as stated by Mosoba and
Kramer (2009), and confirmed from the values calculated using both correction factors (p>0.05). Z score results were satisfactory according
to Thompson et al. (2006). All the mean values for the seven certified FAMEs were ≤2 z, which confirms the homogenity of these results. Related to
the non certified FAMEs, they corresponded to 23 FAMEs, between 10:0 to 13c-22:1, in low percentages, between 0.05–0.9 g·100g−1 total FAME including some positional and geometric isomers.
Table 2. Determined FAME content of the BCR 163 certified reference material and their respective recovery
| FATTY ACIDS |
Certified FAME content
(g·100 g−1 FAME) |
Determined FAME content by ECF
(g·100 g−1 FAME) |
Recovery (%) |
Determined FAME content by TCFr
(g·100 g−1 FAME)
|
Recovery
(%) |
| 14:0# |
2.29±0.04 |
2.16±0.11 |
94.3 |
2.16±0.07 |
94.3 |
| 16:0# |
25.96±0.30 |
25.28±0.65 |
97.4 |
25.42±0.36 |
97.9 |
| 16:1# |
2.58±0.16 |
2.37±0.22 |
91.9 |
2.26±0.22 |
87.6 |
| 18:0# |
18.29±0.17 |
17.80±0.66 |
97.3 |
18.04±0.40 |
98.6 |
| 18:1# |
38.30±0.40 |
37.50±0.69 |
97.9 |
36.52±2.53 |
95.4 |
| 18:2# |
7.05±0.17 |
6.95±0.13 |
98.6 |
6.85±0.16 |
97.2 |
| 18:3 |
0.86±0.14 |
0.78±0.07 |
90.7 |
0.76±0.07 |
88.4 |
| Total |
95.33 |
92.84±1.90 |
97.4 |
92.01±1.96 |
96.5 |
| Mean value ± SD (n = 5). For abbreviations see Table 1.
|
| # Includes, as appropriate, positional and geometrical (i.e. cis/trans) isomers. |
3.4. Fatty Acid composition of commercial fat samplesTOP
The same modified temperature program indicated in 3.1 was applied in the GLC analysis of FAMEs from three commercial fat
samples: a mixture of soybean and sunflower oil (80/20), fish oil and anhydrous butterfat. For the soybean/sunflower mixed
oil, FAMEs were calculated by ECF and TCFr values, to compare both results according to the data discussed in 3.3. This procedure
was not applied for fish oil or anhydrous butterfat (Mossoba and Kramer, 2009) for the reasons previously explained in 2.4 and 3.3. The FAMEs were calculated by the five laboratories, each laboratory
using its own determined ECFs (Table 1). The final results corresponded to mean ± SD (n = 5) of the data reported by each laboratory. To convert g FAME·100 g−1 FAME to g FA·100 g−1 FA and triacylglyceride equivalents % (TAGe) the respective Conversion Factors (CF) tabulated in the AOCS Method Ce 1j-07
(2007) were employed.
3.4.1. Fatty acid composition of refined mixture of soybean and sunflower oilTOP
Eighteen FAs were identified and quantified in the soybean and sunflower oil mixture (80/20), seven SFAs, six MUFAs and five
PUFAs, including cis-trans isomers (Table 3). The FAMEs resolution was good among the eighteen FAMEs, including the close polarity between 18:3 n-3 and 11c-20:1 and the different amount found between both, confirming the high efficiency of the SP-2560 or CP Sil 88 columns and
the good resolution obtained with the modified temperature program. FAs and TAGe values are within the literature range (Firestone,
2006). The content of α-linolenic acid (mean value: 4.7 g%) was low compared with genuine soybean oil at around 7% (Firestone,
2006), confirming the mix with sunflower oil (80:20), commercialized in Argentina, Chile, and declared in the ingredients.
Table 3. Fatty acid composition of refined mixture of soybean and sunflower oil
| FATTY ACIDS |
FAMEECF
(g FAME·100 g−1 FAME)
|
FAECF
(g FA·100g−1 FA)
|
TAGeECF
(%)
|
FAMETCFr
(g FAME·100 g−1 FAME)
|
FATCFr
(g FA·100 g−1 FA)
|
TAGeTCFr
(%)
|
*FACF |
*TAGCF |
| 14:0 |
0.09±0.02 |
0.09±0.02 |
0.09 |
0.09±0.02 |
0.09±0.02 |
0.09 |
0.9421 |
0.9945 |
| 16:0 |
10.50±0.17 |
9.96±0.15 |
10.45 |
10.51±0.19 |
9.96±0.18 |
10.46 |
0.9481 |
0.9950 |
| 17:0 |
0.10±0.02 |
0.09±0.01 |
0.10 |
0.10±0.01 |
0.09±0.01 |
0.10 |
0.9507 |
0.9953 |
| 18:0 |
4.43±0.19 |
4.23±0.18 |
4.41 |
4.49±0.19 |
4.28±0.16 |
4.47 |
0.9530 |
0.9955 |
| 20:0 |
0.15±0.05 |
0.14±0.04 |
0.15 |
0.15±0.05 |
0.14±0.05 |
0.15 |
0.9570 |
0.9959 |
| 22:0 |
0.41±0.04 |
0.39±0.04 |
0.41 |
0.41±0.03 |
0.39±0.03 |
0.41 |
0.9604 |
0.9962 |
| 24:0 |
0.15±0.03 |
0.14±0.03 |
0.15 |
0.15±0.03 |
0.14±0.03 |
0.15 |
0.9633 |
0.9965 |
| ΣTotal SFA |
15.83 |
15.04 |
15.76 |
15.90 |
15.09 |
15.83 |
|
|
| 9c-16:1
|
0.09±0.01 |
0.08±0.02 |
0.09 |
0.08±0.02 |
0.08±0.01 |
0.08 |
0.9477 |
0.9950 |
| 9c -18:1
|
21.63±0.41 |
20.61±0.42 |
21.53 |
21.54±0.44 |
20.52±0.45 |
21.44 |
0.9477 |
0.9950 |
| 11c-18:1
|
1.27±0.04 |
1.21±0.03 |
1.26 |
1.25±0.05 |
1.19±0.05 |
1.24 |
0.9527 |
0.9955 |
| 5c-20:1
|
0.83±0.12 |
0.79±0.13 |
0.83 |
0.77±0.05 |
0.74±0.05 |
0.77 |
0.9568 |
0.9959 |
| 8c-20:1
|
0.68±0.06 |
0.65±0.05 |
0.68 |
0.66±0.06 |
0.63±0.06 |
0.66 |
0.9568 |
0.9959 |
| 11c-20:1
|
0.15±0.02 |
0.14±0.03 |
0.15 |
0.14±0.01 |
0.14±0.01 |
0.14 |
0.9568 |
0.9959 |
| ΣTotal MUFA |
24.65 |
23.48 |
24.54 |
24.44 |
23.30 |
24.33 |
|
|
| 9c,12t-18:2
|
0.55±0.03 |
0.52±0.05 |
0.55 |
0.54±0.03 |
0.51±0.03 |
0.54 |
0.9524 |
0.9954 |
| 9t,12c-18:2
|
0.55±0.06 |
0.52±0.04 |
0.55 |
0.54±0.07 |
0.51±0.07 |
0.54 |
0.9524 |
0.9954 |
| 9c,12c-18:2
|
53.93±0.57 |
51.36±0.84 |
53.68 |
53.56±0.65 |
51.01±0.63 |
53.31 |
0.9524 |
0.9954 |
| 6c,9c,12c-18:3
|
0.11±0.01 |
0.10±0.01 |
0.11 |
0.10±0.02 |
0.10±0.01 |
0.10 |
0.9520 |
0.9954 |
| 9c,12c,15c-18:3
|
4.76±0.29 |
4.53±0.27 |
4.74 |
4.69±0.22 |
4.46±0.21 |
4.67 |
0.9520 |
0.9954 |
| ΣPUFAs cis,cis |
58.80 |
55.99 |
58.53 |
58.35 |
55.57 |
58.08 |
|
|
| ΣPUFAs cis,trans |
1.10 |
1.04 |
1.10 |
1.08 |
1.02 |
1.08 |
|
|
| ΣTotal PUFAs |
59.90 |
57.03 |
59.63 |
59.43 |
56.59 |
59.16 |
|
|
| ΣTotal trans FAs |
1.10 |
1.04 |
1.10 |
1.08 |
1.02 |
1.08 |
|
|
| ΣTotal FAs |
100.38 |
95.55 |
99.93
|
99.77
|
94.98
|
99.32 |
|
|
| Mean value ± SD (n = 5). TAGe, Triacylglycerol equivalents; CF, Conversion factor. For other abbreviations see Table 1.
|
PUFA was the predominant group (60%), with linoleic acid being the main FA with a mean value of 54%. MUFAs were 24%, with
oleic acid being the major constituent with 21%. SFAs represented 15% and the main one was palmitic acid, reaching 11%. The
total g FAME·100 g−1 FAME using ECF or TCFr was up to 99% in both cases, and the conversion to g·100 g−1 FA and TAGe gave values of 95% and 99.9%, respectively, which were considered satisfactory. Thus, calculated ECF or tabulated
TCFr can be used for quantitative purposes in this case. Chromatograms of the FAME separation and emerging time in two zones
of the total FAMEs identified and quantified in this mixture of vegetable oils are presented in Figure 2A: zone 9c-18:1 to 9c,12c-18:2 and Figure 2B: zone 20:0–24:0.
|
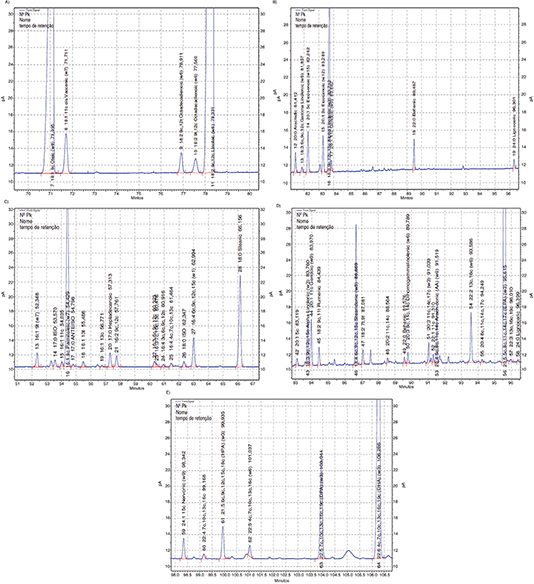 Figure 2. A. Significant part of the chromatogram of mixed soybean oil/Sunflower oil (80/20) showing the zone of FAMEs 9c-18:1 to 9c,12c-18:2 using the proposed temperature program. Figure 2. A. Significant part of the chromatogram of mixed soybean oil/Sunflower oil (80/20) showing the zone of FAMEs 9c-18:1 to 9c,12c-18:2 using the proposed temperature program.
B. Significant part of the chromatogram of mixed soybean oil/sunflower oil (80/20)
showing the zone of FAMEs 20:0 to 24:0 using the proposed temperature program.
C. Significant part of the chromatogram of
fish oil capsules showing the zone of FAMEs 9t-16:1 to 18:0 using the proposed temperature program.
D. Significant part of the chromatogram of fish oil capsules showing
the zone of FAMEs 5c-20:1 to 24:0 using the proposed temperature program.
E. Significant part of the chromatogram of fish oil capsules showing
the zone of FAMEs 24:1 to 4c,7c,10c,13c,16c,19c-22:6, DHA using the proposed temperature program.
|
|
Ratnayake et al., (2006) evaluated the same two polar columns for determining cis-,trans-FAs, SFAs, MUFAs and PUFAs in vegetable and not ruminant animal oils and fats using the AOCS Method Ce 1h-05 (2005), where the FA 21:0 as IS was used. In the present study, 13:0-TAG proposed by the AOCS Method Ce 1j-07 (2007) was utilized.
According to previous experience, 21:0 elutes in the CLA isomers region interfere with the identification and quantification
of these isomers that are present in milk fat and in deodorized fish oils. As these types of samples were also analyzed in
the present study, the use of 13:0-TAG was justified. The absence of 13:0 was checked in the samples analyzed. It is the best
internal standard for butterfat according to Mossoba and Kramer (2009). AOCS Official Methods Ce 2b-11 (2011) and Ce 2c-11 (2011) indicate that in addition to 13:0-TAG, 21:0-TAG and 23:0-TAG as IS can be used for vegetable and fish oils, respectively.
3.4.2. Fatty acid composition of fish oilTOP
In total, sixty three FAMEs were identified and quantified in the fish oil sample (Table 4). They were separated by groups: seventeen SFAs, eighteen MUFAs and twenty eight PUFAs. Fish oil FAs are complex to separate
and to identify, considering their different polarity related to chain length, double bonds from 2 to 6 and positional and
geometric isomers with the same carbon number.
Table 4. Fatty acid composition of fish oil
| FATTY ACIDS |
FAMEECF
(g FAME·100 g−1FAME)
|
FAECF
(g FA·100 g−1 FA)
|
TAGeECF
(%)
|
*FACF |
*TAGCF |
| 12:0 |
0.11±0.02 |
0.10±0.01 |
0.11 |
0.9346 |
0.9937 |
| Iso-13:0
|
0.02±0.01 |
0.02±0.01 |
0.02 |
0.9386 |
0.9941 |
| Iso-14:0
|
0.05±0.01 |
0.05±0.01 |
0.05 |
0.9421 |
0.9945 |
| 14:0 |
7.35±0.43 |
6.92±0.45 |
7.31 |
0.9421 |
0.9945 |
| Iso-15:0
|
0.22±0.02 |
0.21±0.02 |
0.22 |
0.9453 |
0.9948 |
| AnteIso-15:0
|
0.06±0.01 |
0.06±0.01 |
0.06 |
0.9453 |
0.9948 |
| 15:0 |
0.52±0.02 |
0.49±0.03 |
0.52 |
0.9453 |
0.9948 |
| Iso-16:0
|
0.08±0.01 |
0.07±0.01 |
0.08 |
0.9481 |
0.9950 |
| 16:0 |
16.18±1.10 |
15.34±0.86 |
16.10 |
0.9481 |
0.9950 |
| Iso-17:0
|
0.20±0.03 |
0.19±0.03 |
0.20 |
0.9507 |
0.9953 |
| AnteIso-17:0
|
0.08±0.03 |
0.08±0.03 |
0.08 |
0.9507 |
0.9953 |
| 17:0 |
0.43±0.01 |
0.41±0.01 |
0.43 |
0.9507 |
0.9953 |
| Iso-18:0
|
0.14±0.03 |
0.13±0.02 |
0.14 |
0.9530 |
0.9955 |
| 18:0 |
3.21±0.27 |
3.06±0.19 |
3.20 |
0.9530 |
0.9955 |
| 20:0 |
0.19±0.03 |
0.18±0.03 |
0.19 |
0.9570 |
0.9959 |
| 22:0 |
0.16±0.05 |
0.15±0.04 |
0.16 |
0.9602 |
0.9962 |
| 24:0 |
0.03±0.02 |
0.03±0.02 |
0.03 |
0.9630 |
0.9965 |
| ΣTotal SFA |
29.03 |
27.49 |
28.90 |
|
|
| 9c-14:1
|
0.06±0.03 |
0.06±0.03 |
0.06 |
0.9417 |
0.9944 |
| 10c-15:1
|
0.04±0.01 |
0.03±0.02 |
0.04 |
0.9449 |
0.9947 |
| 9t-16:1
|
0.43±0.03 |
0.41±0.04 |
0.43 |
0.9477 |
0.9950 |
| 11c-16:1
|
0.12±0.02 |
0.12±0.02 |
0.12 |
0.9704 |
0.9950 |
| 9c-16:1
|
8.39±0.43 |
8.14±0.42 |
8.35 |
0.9704 |
0.9950 |
| 13t-16:1
|
0.19±0.06 |
0.18±0.05 |
0.19 |
0.9704 |
0.9950 |
| 13c-16:1
|
0.07±0.02 |
0.07±0.02 |
0.07 |
0.9704 |
0.9950 |
| 6t+8t-18:1
|
1.10±0.09 |
1.07±0.12 |
1.10 |
0.9704 |
0.9955 |
| 9t-18:1
|
0.06±0.02 |
0.06±0.01 |
0.06 |
0.9704 |
0.9955 |
| 10t -18:1
|
0.06±0.01 |
0.06±0.01 |
0.06 |
0.9704 |
0.9955 |
| 9c-18:1
|
9.67±0.59 |
9.38±0.40 |
9.63 |
0.9704 |
0.9955 |
| 11c-18:1
|
3.10±0.23 |
3.01±0.16 |
3.09 |
0.9704 |
0.9955 |
| 13c-18:1
|
0.09±0.02 |
0.08±0.02 |
0.09 |
0.9704 |
0.9955 |
| 14c-18:1
|
0.10±0.03 |
0.09±0.03 |
0.10 |
0.9704 |
0.9955 |
| 5c-20:1
|
0.14±0.03 |
0.13±0.02 |
0.14 |
0.9520 |
0.9959 |
| 11c-20:1
|
0.75±0.07 |
0.72±0.06 |
0.75 |
0.9568 |
0.9959 |
| 11c-22:1
|
0.13±0.02 |
0.12±0.02 |
0.13 |
0.9600 |
0.9962 |
| 15c-24:1
|
0.35±0.02 |
0.34±0.02 |
0.35 |
0.9628 |
0.9965 |
| ΣMUFA cis |
23.01 |
22.29 |
22.92 |
|
|
| ΣMUFA trans |
1.84 |
1.78 |
1.84 |
|
|
| ΣTotal MUFA |
24.85 |
24.07 |
24.76 |
|
|
| 9c,12c-16:2
|
0.29±0.06 |
0.27±0.06 |
0.29 |
0.9473 |
0.9950 |
| 6c,9c,12c-16:3
|
0.19±0.03 |
0.18±0.03 |
0.19 |
0.9469 |
0,9950 |
| 7c,10c,13c-16:3
|
0.08±0.01 |
0.08±0.01 |
0.08 |
0.9469 |
0.9950 |
| 3c,6c,9c,12c-16:4
|
0.08±0.02 |
0.08±0.02 |
0.08 |
0.9465 |
0.9950 |
| 4c,7c,10c,13c-16:4
|
0.13±0.03 |
0.12±0.03 |
0.13 |
0.9465 |
0.9950 |
| 6c,9c,12c,15c-16:4
|
1.04±0.13 |
0.98±0.14 |
1.03 |
0.9465 |
0.9950 |
| 9t,12t-18:2
|
0.08±0.01 |
0.08±0.02 |
0.08 |
0.9524 |
0.9954 |
| 8c,11c-18:2
|
0.15±0.04 |
0.14±0.04 |
0.15 |
0.9524 |
0.9954 |
| 9c,12c-18:2
|
2.04±0.12 |
1.94±0.14 |
2.03 |
0.9524 |
0.9954 |
| 11c,14c-18:2
|
0.36±0.05 |
0.34±0.05 |
0.36 |
0.9524 |
0.9954 |
| 9c,11t-18:2
|
0.28±0.03 |
0.27±0.03 |
0.28 |
0.9524 |
0.9954 |
| 7t,9t-18:2
|
0.24±0.03 |
0.23±0.03 |
0.24 |
0.9524 |
0.9954 |
| 6c,9c,12c-18:3
|
0.20±0.02 |
0.19±0.03 |
0.20 |
0.9520 |
0.9954 |
| 9c,12c,15c-18:3
|
0.71±0.08 |
0.68±0.07 |
0.71 |
0.9520 |
0.9954 |
| 6c,9c,12c,15c-18:4
|
2.34±0.34 |
2.23±0.36 |
2.33 |
0.9517 |
0.9954 |
| 11c,14c-20:2
|
0.17±0.03 |
0.16±0.03 |
0.17 |
0.9565 |
0.9958 |
| 8c,11c,14c-20:3
|
0.11±0.03 |
0.11±0.03 |
0.11 |
0.9562 |
0.9958 |
| 11c,14c,17c-20:3
|
0.32±0.01 |
0.31±0.02 |
0.32 |
0.9562 |
0.9958 |
| 5c,8c,11c,14c-20:4
|
0.84±0.06 |
0.80±0.07 |
0.84 |
0.9560 |
0.9958 |
| 8c,11c,14c,17c-20:4
|
0.05±0.01 |
0.04±0.01 |
0.05 |
0.9560 |
0.9958 |
| 5c,8c,11c,14c,17c-20:5
|
16.85±0.99 |
16.10±0.87 |
16.78 |
0.9557 |
0.9958 |
| 4c,7c,10c,13c,16c-21:5
|
0.62±0.07 |
0.59±0.08 |
0.62 |
0.9578 |
0.9961 |
| 13c,16c-22:2
|
0.80±0.12 |
0.77±0.12 |
0.80 |
0.9600 |
0.9962 |
| 13c,16c,19c-22:3
|
0.04±0.01 |
0.04±0.02 |
0.04 |
0.9598 |
0.9961 |
| 7c,10c,13c,16c-22:4
|
0.09±0.01 |
0.09±0.02 |
0.09 |
0.9595 |
0.9961 |
| 4c,7c,10c,13c,16c-22:5
|
0.31±0.06 |
0.30±0.06 |
0.31 |
0.9593 |
0.9961 |
| 7c,10c,13c,16c,19c-22:5
|
2.19±0.14 |
2.10±0.14 |
2.18 |
0.9593 |
0.9961 |
| 4c,7c,10c,13c,16c,19c-22:6
|
10.93±0.79 |
10.48±0.75 |
10.89 |
0.9590 |
0.9961 |
| ΣPUFAs cis,cis |
40.93 |
39.12 |
40.78 |
|
|
| ΣPUFAs cis,trans |
0.60 |
0.58 |
0.60 |
|
|
| ΣTotal PUFAs |
41.53 |
39.70 |
41.38 |
|
|
| ΣTotal trans FAs |
2.44 |
2.36 |
2.44 |
|
|
| ΣTotal FAs |
|
|
|
|
|
|
95.41 |
91.26 |
95.04 |
|
|
| Mean value ± SD (n=5). For abbreviations see Tables 1 and 3.
|
For the identification of FAMEs, different standards and literature data were consulted. The standard GLC 463 has 52 FAMEs,
34 of them being present in the fish oil analyzed (65%). Santercole et al. 2012, using direct GLC and fractions obtained with silver ions, quantified the FAMEs in Menhaden oil (MO) and Sparus aurata fish oil using an SP-2560 100 m capillary column. The temperature program takes 110.3 min, practically the same time as the
program developed in this work (110 min). GLC 463 for FAME identification was also used. A good agreement was found in the
elution order and FAME identification between both studies, even though they employed different samples.
Chromatograms of the FAME in three zones of the total FAMEs identified and quantified in the fish oil sample analyzed are
presented. Figure 2C shows 9t-16:1 to 18:0, Figure 2D: 5c-20:1 to 24,0 and Figure 2E: 15c-24,1 to 22,6 DHA. It is important to mention that the FAME elution and identification presented in Figure 2D and in Figure 2E follow practically the same order indicated for MO in Santercole el al., 2012, who concluded that better results were obtained using the SP-2560 capillary column than the Supercowax −10, 30 m
indicated in the Official AOCS Method Ce 1i-07 (AOCS, 2007a) for marine oils. Some small peaks were not identified, their retention times not fitting available standards.
The PUFA group achieved the major percentage of the total FAs (40%), 20:5 n-3 and 22:6 n-3 being the most abundant with 16.9%
and 10.9%, respectively. This FA composition agrees with data published for fish oils, where SFAs and MUFAs are in equivalent
proportion and PUFAs are the prevalent group (SFAs: MUFAs: PUFAs/ 1.0: 0.9: 1.4) (Romero et al., 1996; Ackman, 1998; Firestone, 2006; Mendez et al., 2010). It is worth mentioning that the temperature program is key in the elution order of the FAMEs with close polarity and different
structure; any small change in the temperature program used can produce overlapping between 9c,12c,15c-18:3 with 11c-20:1; 11c-22:1 with 20:4 n-6, 20:5 n-3 with 24:0 or changes in the elution order. The modified temperature program used made it possible
to identify and properly quantify a total of sixty three FAMEs. The mean value obtained among the five laboratories of 95.41
g FAME·100 g−1 of FAMEs is considered satisfactory, Table 4.
3.4.3. Fatty acid composition of anhydrous butterfatTOP
Anhydrous butterfat is another complex animal fat to analyze FA composition by GLC due the large number of FAs with wide carbon
chain numbers and different isomers, starting with butyric acid (4:0) as shown in Figure 3A. Numerous papers have been published in recent years related to butterfat FA analysis, with a special mention for the separation
and identification of trans isomers. Differences in quantity and distribution of 18:1 trans-isomers formed in ruminant and industrial processing constitute an interesting health issue, as has been described by Mossoba and Kramer (2009). The 11t-18:1 trans- vaccenic acid is the most abundant FA isomer in ruminant fats, as can be observed in Figure 3B. The rumenic acid (9c,11t-18:2), the main CLA isomer present in milk fat and very important FA due to its biological role (Roach et al., 2000; Dionisi et al., 2002; Kramer et al., 2004; Destaillats et al., 2007) is clearly identified in Figure 3C.
|
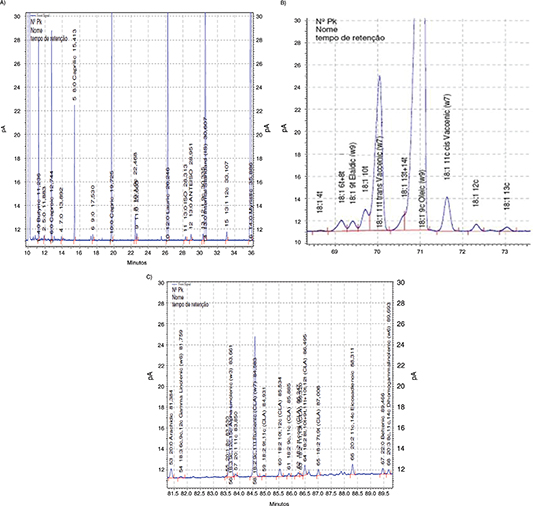 Figure 3. Significant parts of the chromatogram of anhydrous butterfat showing the zones of FAMEs: A. 4:0 to 14:0, B. 4t-18:1 to 13c-18:1 and C. 20:0 to 11c,14c,17c-20:3 using the proposed temperature program. Figure 3. Significant parts of the chromatogram of anhydrous butterfat showing the zones of FAMEs: A. 4:0 to 14:0, B. 4t-18:1 to 13c-18:1 and C. 20:0 to 11c,14c,17c-20:3 using the proposed temperature program.
|
|
Each laboratory reported the mean value for each FAME identified, expressed as g FAME·100g−1 FAMEs at a final mean value ± SD (n = 5) for each FAME and the results are presented in Table 5.
Table 5. Fatty acid composition of anhydrous butterfat
| FATTY ACIDS |
FAMEECF
(g FAME·100 g−1 FAME)
|
FAECF
(g FA·100 g−1 FA)
|
TAGeECF
(%)
|
FACF |
TAGCF |
| 4:0 |
2.33±0.73 |
1.99±0.62 |
2.30 |
0.8623 |
0.9868 |
| 5:0 |
0.06±0.05 |
0.05±0.05 |
0.06 |
0.8792 |
0.9884 |
| 6:0 |
1.89±0.31 |
1.69±0.28 |
1.87 |
0.8922 |
0.9897 |
| 7:0 |
0.03±0.00 |
0.03±0.00 |
0.03 |
0.9027 |
0.9907 |
| 8:0 |
1.24±0.11 |
1.13±0.13 |
1.23 |
0.9114 |
0.9915 |
| 9:0 |
0.03±0.01 |
0.03±0.01 |
0.03 |
0.9186 |
0.9922 |
| 10:0 |
2.81±0.27 |
2.60±0.26 |
2.79 |
0.9247 |
0.9928 |
| 11:0 |
0.06±0.02 |
0.06±0.01 |
0.06 |
0.9296 |
0.9933 |
| 12:0 |
3.34±0.43 |
3.12±0.42 |
3.32 |
0.9346 |
0.9937 |
| Iso-13:0
|
0.05±0.01 |
0.05±0.01 |
0.05 |
0.9386 |
0.9941 |
| Anteiso-13:0
|
0.07±0.01 |
0.07±0.01 |
0.07 |
0.9386 |
0.9941 |
| 14:0 |
10.74±0.58 |
10.12±0.68 |
10.68 |
0.9421 |
0.9945 |
| Anteiso-15:0
|
0.59±0.07 |
0.56±0.07 |
0.59 |
0.9453 |
0.9948 |
| 15:0 |
1.18±0.14 |
1.12±0.14 |
1.17 |
0.9453 |
0.9948 |
| Iso-16:0
|
0.26±0.03 |
0.25±0.03 |
0.26 |
0.9481 |
0.9950 |
| 16:0 |
26.74±1.01 |
25.35±1.05 |
26.61 |
0.9481 |
0.9950 |
| Iso-17:0 |
0.09±0.01 |
0.08±0.02 |
0.09 |
0.9507 |
0.9953 |
| 17:0 |
0.62±0.06 |
0.60±0.06 |
0.62 |
0.9507 |
0.9953 |
| 18:0 |
11.27±0.83 |
10.74±0.73 |
11.22 |
0.9530 |
0.9955 |
| 19:0 |
0.08±0.03 |
0.08±0.02 |
0.08 |
0.9551 |
0.9957 |
| 20:0 |
0.15±0.03 |
0.14±0.03 |
0.15 |
0.9570 |
0.9959 |
| 22:0 |
0.07±0.02 |
0.07±0.02 |
0.07 |
0.9602 |
0.9962 |
| 24:0 |
0.05±0.01 |
0.04±0.01 |
0.05 |
0.9630 |
0.9965 |
| ΣTotal SFA |
63.75 |
59.97 |
63.40 |
|
|
| 9c-10:1
|
0.24±0.05 |
0.22±0.04 |
0.24 |
0.9239 |
0.9927 |
| 9c-12:1
|
0.08±0.02 |
0.08±0.02 |
0.08 |
0.9339 |
0.9937 |
| 12c-13:1
|
0.12±0.02 |
0.11±0.02 |
0.12 |
0.938 |
0.9941 |
| 9t-14:1
|
0.36±0.16 |
0.34±0.15 |
0.36 |
0.9416 |
0.9944 |
| 9c-14:1
|
0.88±0.10 |
0.83±0.09 |
0.88 |
0.9416 |
0.9944 |
| 9t-16:1
|
0.13±0.03 |
0.12±0.02 |
0.13 |
0.9477 |
0.9950 |
| 11t-16:1
|
0.38±0.17 |
0.36±0.16 |
0.38 |
0.9477 |
0.9950 |
| 9c-16:1
|
1.26±0.24 |
1.19±0.23 |
1.25 |
0.9477 |
0.9950 |
| 10c-16:1
|
0.50±0.06 |
0.47±0.22 |
0.50 |
0.9477 |
0.9950 |
| 11c-16:1
|
0.16±0.04 |
0.15±0.04 |
0.16 |
0.9477 |
0.9950 |
| 9c-17:1
|
0.23±0.04 |
0.22±0.04 |
0.23 |
0.9503 |
0.9952 |
| 4t-18:1
|
0.04±0.01 |
0.04±0.01 |
0.04 |
0.9527 |
0.9955 |
| 6t-8t-18:1
|
0.33±0.06 |
0.31±0.06 |
0.33 |
0.9527 |
0.9955 |
| 9t-18:1
|
0.26±0.02 |
0.25±0.01 |
0.26 |
0.9527 |
0.9955 |
| 10t-18:1
|
0.57±0.03 |
0.54±0.04 |
0.57 |
0.9527 |
0.9955 |
| 11t-18:1
|
2.39±0.12 |
2.28±0.11 |
2.38 |
0.9527 |
0.9955 |
| 13t-14t-18:1
|
0.22±0.06 |
0.21±0.06 |
0.22 |
0.9527 |
0.9955 |
| 9c-18:1
|
21.80±0.98 |
20.77±1.02 |
21.70 |
0.9527 |
0.9955 |
| 11c-18:1
|
0.90±0.05 |
0.86±0.04 |
0.90 |
0.9527 |
0.9955 |
| 12c-18:1
|
0.27±0.02 |
0.26±0.02 |
0.27 |
0.9527 |
0.9955 |
| 13c-18:1
|
0.08±0.02 |
0.08±0.02 |
0.08 |
0.9527 |
0.9955 |
| 14c-18:1
|
0.06±0.00 |
0.06±0.01 |
0.06 |
0.9527 |
0.9955 |
| 16t-18:1
|
0.49±0.07 |
0.47±0.07 |
0.49 |
0.9527 |
0.9955 |
| 15c-18:1
|
0.18±0.07 |
0.17±0.07 |
0.18 |
0.9527 |
0.9955 |
| 8c-20:1
|
0.05±0.01 |
0.05±0.01 |
0.05 |
0.9568 |
0.9959 |
| 11c-20:1
|
0.01±0.01 |
0.01±0.00 |
0.01 |
0.9568 |
0.9959 |
| 15c-24:1
|
0.04±0.01 |
0.03±0.01 |
0.04 |
0.9628 |
0.9965 |
| ΣMUFA cis |
26.86 |
25.56 |
26.75 |
|
|
| ΣMUFA trans |
5.17 |
4.92 |
5.16 |
|
|
| ΣTotal MUFA |
32.03 |
30.48 |
31.91 |
|
|
| 9t,12t-18:2
|
0.11±0.01 |
0.11±0.02 |
0.11 |
0.9524 |
0.9954 |
| 9t,13c-18:2 + 8t,12c-18:2
|
0.15±0.01 |
0.14±0.01 |
0.15 |
0.9524 |
0.9954 |
| 9c,12t-18:2 + 8t,13c-18:2
|
0.05±0.03 |
0.05±0.03 |
0.05 |
0.9524 |
0.9954 |
| 9t,12c-18:2
|
0.52±0.31 |
0.50±0.35 |
0.52 |
0.9524 |
0.9954 |
| 11t,15c-18:2
|
0.08±0.02 |
0.08±0.02 |
0.08 |
0.9524 |
0.9954 |
| 9c,12c-18:2
|
2.11±0.20 |
2.01±0.21 |
2.10 |
0.9524 |
0.9954 |
| 9c,15c-18:2
|
0.08±0.02 |
0.08±0.03 |
0.08 |
0.9524 |
0.9954 |
| 6c,9c,12c-18:3
|
0.03±0.01 |
0.03±0.01 |
0.03 |
0.9520 |
0.9954 |
| 9c,12c,15c-18:3
|
0.75±0.22 |
0.71±0.21 |
0.75 |
0.9520 |
0.9954 |
| 9c,11t-18:2
|
1.19±0.43 |
1.13±0.42 |
1.18 |
0.9524 |
0.9954 |
| 9t,11c-18:2
|
0.02±0.00 |
0.02±0.00 |
0.02 |
0.9524 |
0.9954 |
| 10t,12c-18:2
|
0.05±0.03 |
0.05±0.03 |
0.05 |
0.9524 |
0.9954 |
| 9c,11c-18:2
|
0.03±0.01 |
0.03±0.01 |
0.03 |
0.9524 |
0.9954 |
| 11t,13t-18:2
|
0.03±0.01 |
0.03±0.01 |
0.03 |
0.9524 |
0.9954 |
| 8t,10t + 9t,11t + 10t,12t-18:2
|
0.08±0.01 |
0.08±0.01 |
0.08 |
0.9524 |
0.9954 |
| 7t,9t-18:2
|
0.03±0.02 |
0.03±0.02 |
0.03 |
0.9524 |
0.9954 |
| 11c,14c-20:2
|
0.10±0.02 |
0.09±0.02 |
0.10 |
0.9565 |
0.9958 |
| 8c,11c,14c-20:3
|
0.07±0.02 |
0.06±0.02 |
0.07 |
0.9562 |
0.9958 |
| 11c,14c,17c-20:3
|
0.06±0.05 |
0.06±0.05 |
0.06 |
0.9560 |
0.9958 |
| 13c,16c-22:2
|
0.04±0.02 |
0.04±0.02 |
0.04 |
0.9600 |
0.9962 |
| 5c,8c,11c,14c-20:4
|
0.04±0.02 |
0.04±0.02 |
0.04 |
0.9560 |
0.9958 |
| 5c,8c,11c,14c,17c-20:5
|
0.06±0.03 |
0.06±0.03 |
0.06 |
0.9557 |
0.9958 |
| 7c,10c,13c,16c,19c-22:5
|
0.08±0.01 |
0.08±0.02 |
0.08 |
0.9593 |
0.9961 |
| ΣPUFAs cis,cis |
3.45 |
3.29 |
3.44 |
|
|
| ΣPUFAs cis,trans |
2.31 |
2.22 |
2.30 |
|
|
| ΣTotal PUFAs |
5.76 |
5.51 |
5.74 |
|
|
| ΣTotal trans FAs |
7.48 |
7.14 |
7.46 |
|
|
| ΣTotal FAs |
101.54 |
95.96 |
101.05 |
|
|
| Mean value ± SD (n = 5). For abbreviations see Tables 1 and 3.
|
Seventy-three FAMEs were identified and quantified. They were organized by groups, SFAs, MUFAs and PUFAs, including their
respective positional and geometric isomers, as shown in Table 5. A very good resolution with the modified temperature program among all short chains FAs was obtained, 4:0 was completely
separated from the solvent, even and odd FAs, iso-, anteiso-, cis- and trans-MUFA isomers, PUFAs, CLA isomers, were also well resolved (Figure 3).
The SFAs represented more than 60%, MUFAs about 32% and PUFAs the lowest percentage at 5.7%, which is characteristic of ruminant
fats. Seppänen-Laakso et al. (2002) indicated levels of 70% for SFAs, 25% for MUFAs, and lower amounts of 11t- and 11c-18:1 isomers. Kramer et al. (2008) showed a wide variation in SFAs (47.03–70.51%), 11t-18:1 (2.68–21.33%), total trans-18:1 (2.84–23.16%) and cis,trans-18:2 (0.77–1.90%).
Due to the complexity of CLA separation, combined techniques have been used in dairy and beef fats (Kramer et al., 2004). Other authors, lowering the oven temperature, improved the trans-18:1 isomer resolution, using previous AgNO3-TLC fractionation and Ag-SPE cartridges (Seppänen-Laakso et al., 2002). Related to FAME preparation, Mossoba and Kramer (2009) indicated that the derivative procedure with BF3 can produce isomers of rumenic acid. In this study, after rumenic acid, six CLA FAME isomers were detected in very small
amounts of 0.02–0.05%, in agreement with those described in the AOCS Method Ce 1j-07 (AOCS, 2007b).
Rozema et al. (2008) proposed modifications to the AOAC Method 996.06 (AOAC, 2005) for determining trans-FAs in butter, combining it with the AOCS Method Ce1h-05 (AOCS, 2005), using the same high polar capillary columns, but applying an external standard relative to 11:0 as IS to identify and quantify
trans-18:1 and trans-18:2 isomers with a different temperature program. By this technique trans-isomers were not completely resolved and regions of trans-isomers were quantified as grouped peaks, and CLA isomers were not considered. In our work, 11:0 was identified and quantified
in the butterfat sample; therefore, 13:0-TAG as IS was used, according to the AOCS Method Ce 1j-07 (2007b).
Rozema et al. (2008) reported total trans-18:1 isomers at 3.37% and trans-18:2 isomers at 0.68% for butter. These values were much lower than those determined in this study, because all the trans-FA identified in the butterfat sample were quantified separately.
Kramer et al. (2008), considering that the recommended Ag-ion separation to solve the overlapping of isomers in milk fat is time-consuming and
not practical for routine analysis, assayed a GLC method using a CP Sil 88 column, combining the results of two temperature
programs with a plateau at 175 °C and another at 150 °C. The results at 175 °C were used as a quantitative reference and those
obtained at 150 °C were used to correct the data. Both programs together are much longer than the modified temperature program
developed in our study. The results obtained for the principal SFAs, MUFAs, PUFAs, and their respective cis- and trans-isomers present in this anhydrous milk fat sample were in general, within the range reported in the literature.
4. CONCLUSIONSTOP
This work shows the advantages of the improved temperature program developed and applied among the five laboratories for FA
analysis in some commercial fat samples compared with the one indicated in the AOCS Method Ce-1j 07 (AOCS, 2007b). Specifically, it is reproducible and allows a clear resolution of FAs, especially 4:0 from the solvent, trans-18:1 from cis-18:1, 20:1 isomers from 18:3 n-3, 22:1 from 20:4 n-6, 20:5 n-3 from 24:0, and the main CLA isomers. Despite the fact that
this modified temperature program is more time consuming than other programs, this proposal could be a good alternative to
determine FA in samples such as soybean and sunflower mixed oil, and more complex ones such as fish oils and butterfat. In
addition, for the FAME quantification of fats and oils containing a wide spectrum of FAs and their isomers, such as fish oils
and ruminant dairy fats, the use of their determined correction factor ECF for each FAME, as well as the use of 13:0-TAG as
IS, is recommended. In the case of edible oils like soybean and sunflower mixed oil, either the ECF or TCFr can be used as
correction factor for the quantification of FAs. The temperature program proposed provides a good alternative for FA quantification
for different purposes such as nutritional labeling, quality control, and research.
ACKNOWLEDGEMENTSTOP
This work was financed by Universidad Nacional del Litoral-Cursos de Acción para la Investigación y Desarrollo (CAI + D 2009)
- Secretaría de Ciencia y Técnica- UNL- Argentina and Agencia Nacional de Promoción Científica, Tecnológica y de Innovación
(ANPCyT - FITS # 001/2010) Argentina. This research was also supported with funds from CAPES, Brasil; Instituto de Nutrição
Universidade Federal do Rio de Janeiro, RJ, Brasil; Faculdade de Ciências Farmacêuticas Universidade de San Paulo, SP, Brasil;
INCIENSA, San José, Costa Rica and Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile, Santiago, Chile. Finally,
the authors wish to thank CYTED (Programa de Ciencia y Tecnología para el Desarrollo – Ministerio Español de Ciencia y Tecnología–BFI2002-00218)
for the financial support of the Red Temática 208RT0343, where this research was integrated.
REFERENCESTOP
| ○ |
AOAC. 2005. Official method 996.06. Official methods of analysis of AOAC International, 18th Ed. AOAC INTERNATIONAL, Gaithersburg, MD.
|
| ○ |
AOCS. 2005. Official method Ce 1h-05. Official methods and recommended practices of the AOCS. 6th Ed. AOCS, Champaign, IL, USA.
|
| ○ |
AOCS. 2007a. Official method Ce 1i-07. Official methods and recommended practices of the AOCS. 6th Ed. AOCS. Champaign, IL, USA.
|
| ○ |
AOCS. 2007b. Official method Ce 1j-07. Official methods and recommended practices of the AOCS. 6th Ed. AOCS, Champaign, IL, USA.
|
| ○ |
AOCS. 2011. Official Method Ce 2b-11. Official methods and recommended practices of the AOCS. 6th Ed. AOCS, Champaign, IL, USA.
|
| ○ |
AOCS. 2011. Official Method Ce 2c-11. Official methods and recommended practices of the AOCS. 6th Ed. AOCS, Champaign, IL, USA.
|
| ○ |
Ackman RG. 1998. The year of fish oil. Chem. Indus. 3, 139–145.
|
| ○ |
Christie WW, Dobson G, Adlof RO. 2007. A practical guide to the isolation, analysis and identification of conjugated linoleic
acid. Lipids 42, 1073–1084. http://dx.doi.org/10.1007/s11745-007-3107-8.
|
| ○ |
Destaillats F, Golay PA, Joffre F, Wispelaere M, Hug B, Giuffrida F, Fauconnot L, Dionisi F. 2007. Comparison of available
analytical methods to measure trans-octadecenoic acid isomeric profile and content by gas-liquid chromatography in milk fat.
J. Chromatog. A 1145, 222–228. http://dx.doi.org/10.1016/j.chroma.2007.01.062.
|
| ○ |
Dionisi F, Golay PA, Fay LB. 2002. Influence of milk fat presence on the determination of trans fatty acids in fats used for
infant formulae. Anal. Chim. Acta 465, 395–407. http://dx.doi.org/10.1016/S0003-2670(02)00126-5.
|
| ○ |
Firestone D. 2006. Physical and chemical characteristics of oils, fat and waxes. 2nd Ed. AOCS Press. Champaign, IL, USA. |
| ○ |
International Standard ISO 5509, 2000 (E). Animal and vegetable fats and oils-Preparation of methyl esters of fatty acids.
2nd Ed. International Organization for Standardization, Geneva, Switzerland.
|
| ○ |
Kramer JK, Sehat N, Dugan ME, Mossoba MM, Yurawecz MP, Roach JA, Eulitz K, Aalhus JL, Schaefer AL, Ku Y. 1998. Distribution
of conjugated linoleic acid (CLA) isomers in tissue lipid classes of pigs fed a commercial CLA mixture determined by gas chromatography
and silver ion-high-performance liquid chromatography. Lipids 33, 549–558. http://dx.doi.org/10.1007/s11745-998-0239-1.
|
| ○ |
Kramer JKG, Cruz-Hernández C, Deng Z, Zou J, Jahreis G, Dugan MER. 2004. Analysis of conjugated linoleic acid and trans 18:1
isomers in synthetic and animal products. Am. J. Clin. Nutr. 79, 1137S–1145S.
|
| ○ |
Kramer JKG, Hernández M, Cruz-Hernández C, Kraft J, Dugan MER. 2008. Combining results of two GC separations partly achieves
determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers in milk fat as demonstrated using Ag-ion SPE
fractionation. Lipids 43, 259–273. http://dx.doi.org/10.1007/s11745-007-3143-4.
|
| ○ |
Manzano P, Diego JC, Nozal MJ, Bernal JL, Bernal J. 2012. Gas chromatography-mass spectrometry approach to study fatty acid
profiles in fried potato crisps. J. Food Compos. Anal. 28, 31–39. http://dx.doi.org/10.1016/j.jfca.2012.07.003.
|
| ○ |
Mendez C, Masson L, Jiménez P. 2010. Estabilización de aceites de pescado por medio de antioxidantes naturales. Aceites Grasas 80, 270–278.
|
| ○ |
Mossoba MM, Kramer JKG. 2009. Official methods for the determination of trans fat. 2nd Edition, AOCS Press, Urbana, IL. 1–74.
|
| ○ |
Ratnayake WMN. 2004. Overview of methods for the determination of trans fatty acids by gas chromatography, silver-ion thin-layer
chromatography, silver-ion liquid chromatography, and gas chromatography/mass spectrometry. J. AOAC Int. 87, 523–539.
|
| ○ |
Ratnayake WMN, Hansen S, Keneddy MP. 2006. Evaluation of the CP Sil 88 and SP-2560 GC columns used in the recently approved
AOCS Official Method Ce 1h-05: determination of cis-, trans-, saturated, monounsaturated, and polyunsaturated fatty acids
in vegetable or non-ruminant animal oils and fats by capillary GLC method. J. AOAC Int. 89, 475–488.
|
| ○ |
Roach JA, Yurawecz MP, Kramer JK, Mossoba MM, Eulitz K, Ku Y. 2000. Gas chromatography-high resolution selected-ion mass spectrometric
identification of trace 21:0 and 20:2 fatty acids eluting with conjugated linoleic acid isomers. Lipids 35, 797–802. http://dx.doi.org/10.1007/s11745-000-0588-9.
|
| ○ |
Romero N, Robert P, Masson L, Luck C, Buchmann L. 1996. Composición en ácidos grasos y aporte de colesterol de conservas de
jurel, sardina, salmón y atún al natural. Arch. Latinoam. Nutr. 46, 75–77.
|
| ○ |
Rozema B, Mitchell B, Winters D, Kohn A, Sullivan D, Meinholz E. 2008. Proposed modifications to AOAC 996.06, optimizing the
determination of trans fatty acids: presentation of data. J. AOAC Int. 91, 92–97.
|
| ○ |
Ruiz-Rodriguez A, Reglero G, Ibañez E. 2010. Recent trends in the advanced analysis of bioactive fatty acids. J. Pharm. Biomed. Anal. 51, 305–326. http://dx.doi.org/10.1016/j.jpba.2009.05.012.
|
| ○ |
Santercole V, Delmonte P, Kramer J. 2012. Comparison of separations of fatty acids from fish products using a 30-m Supelcowax-10
and a 100-m SP-2560 column. Lipids 47, 329–344. http://dx.doi.org/10.1007/s11745-011-3645-y.
|
| ○ |
Seppänen-Laakso T, Laakso I, Hiltunen R. 2002. Analysis of fatty acids by gas chromatography, and its relevance to research
on health and nutrition. Anal. Chim. Acta 465, 39–62. http://dx.doi.org/10.1016/S0003-2670(02)00397-5.
|
| ○ |
Smith S, Hansen SL. 2008. Statistical analysis of the collaborative study in support of the Official Method AOCS Ce 1i-07:
Determination of saturated, cis-monounsaturated and cis-polyunsaturated fatty acids in marine and other oils containing long
chain polyunsaturated fatty acids by capillary GLC. J. AOCS Int. 85, 901–909.
|
| ○ |
Thompson M, Ellison SL, Wood R. 2006. The international harmonized protocol for the proficiency testing of analytical chemistry
laboratories (IUPAC Technical Report). Pure Appl. Chem. 78, 161–163. http://dx.doi.org/10.1351/pac200678010145.
|
| ○ |
Van de Voort FR, Ghetler A, García-González DL, Li YD. 2008. Perspectives on Quantitative Mid-FTIR Spectroscopy in Relation
to Edible Oil and Lubricant Analysis: Evolution and Integration of Analytical Methodologies. Food Anal. Methods 1, 153–163. http://dx.doi.org/10.1007/s12161-008-9031-6.
|
Figure 1. A. Representative chromatogram of GLC 463 Certified Reference Standard using the AOCS Official Method Ce 1j-07. B. Representative
chromatogram of GLC 463 Certified Reference Standard using the proposed temperature program (see paragraph 3.1 for operating
conditions).