Changes in the sterol compositions of milk thistle oil (Silybium marianum L.) during seed maturation
S. Harrabia,*,
S. Curtisb,
F. Hayeta and P.M. Mayerb
aBiochemistry Department, Faculty of Medicine Tunis, University of Tunis El-Manar, Tunisia
bChemistry Department, University of Ottawa, Ottawa, ONK1N6N5, Canada
*Corresponding author: sawsemtahar@yahoo.fr
| |
SUMMARY
In this study, the total lipid content and sterol compositions were determined during the development of milk thistle seeds.
The oil content increased to a maximum value of 36±1.7% and then declined to reach a value of 30.5±0.9% at full maturity.
The sterol content of milk thistle seeds was affected by the ripening degree of the seeds. At the early stages of seed maturation,
Δ7-stigmastenol was the most abundant sterol followed by β-sitosterol. However, at full maturity, β-sitosterol
was the most predominant sterol (46.50±0.8%). As the seed developed, campesterol and stigmasterol amounts increased, while
Δ7-avenasterol content decreased. It can be concluded that milk thistle seed oil has a characteristic sterol pattern comparable
to the ones elucidated for olive oil and corn oil. The extracted oil from milk thistle seeds is rich in phytosterols and could
be used in food preparation and human nutrition.
|
| |
RESUMEN
Cambios en la composición de esteroles del aceite de cardo mariano (Silybium marianum L.) durante la maduración de la semilla. En este estudio se determinaron la composición de lípidos totales y esteroles durante el desarrollo de semillas de cardo
mariano. El contenido de aceite incrementó a un valor máximo de 36±1,7% y posteriormente disminuyó hasta alcanzar un valor
de 30,5±0,9% cuando la maduración fue completa. El contenido de esteroles de las semillas de cardo mariano se ve afectado
por el grado de maduración de las semillas. En las primeras etapas de la maduración de las semillas el Δ7-estigmastenol
fué el esterol más abundante, seguido de β-sitosterol. Sin embargo en plena madurez, β-sitosterol fue el
esterol predominante (46,50±0,8%). A medida que las semillas se desarrollan las cantidades de campesterol y estigmasterol
aumentan, mientras que el contenido Δ7-avenasterol disminuye. Se puede concluir que el aceite de semillas de cardo
mariano tiene un patrón característico de esteroles en comparación con lo especificado para los aceites de oliva y de maíz.
El aceite extraído de las semillas del cardo mariano es rica en fitoesteroles y podría ser utilizado en la preparación de
alimentos y en nutrición humana.
|
1. INTRODUCTIONTOP
Phytosterols are minor components of vegetable oils and form a major proportion of the unsaponifiables (Azadmard-Damirchi
et al., 2005). The individual sterols and their relative proportions can be used to determine the identity of the oil and to detect adulterations.
It has been reported that conventional refining does not significantly affect sterol composition. Phytosterol contents in
vegetables are known to vary due to different factors such as variety, season, extraction and other technological procedures
(Li et al., 2007; Cercaci et al., 2007).
Furthermore, Phytosterols are known to lower serum low-density lipoprotein (LDL) cholesterol levels by reducing intestinal
cholesterol absorption (Miettinenet et al., 1995). Clinical studies confirmed that phytosterols have hypocholesterolemy, anti-inflammatory and anti-carcinogenic effects (Awad
et al., 2007; Ronco et al., 1999; Berges et al., 1995). Therefore, phytosterols have been added to several functional food products such as yoghurt, milk (Lagarda et al., 2006), and some vegetable oils (Ntanios, 2001). These types of products are now available on the market and have been scientifically proven to lower blood LDL cholesterol
by around 10–15% as part of a healthy diet (Jones et al., 2000).
Milk thistle is an important medicinal crop in Europe and has recently become more significant in North America (Zheljazkov
et al., 2006). The medicinal compounds of value are found in the seeds of the plant. In Tunisia, milk thistle is a common wild plant,
which grows in many regions, and the people from some regions, particularly in the center areas, eat the seeds of this plant.
Milk thistle seeds have been used for more than 2000 years to treat liver diseases (Karkanis et al., 2011). These seeds contain silymarin (Engelberth et al., 2008) and 25% (w/w) oil (Wallace et al., 2005). The oil has to be removed from the seeds prior to the extraction of silymarin. Therefore, it is a by-product of silymarin
production. The oil extracted from these seeds can be used as a cure for many diseases including viral hepatitis and cirrhosis
(Fadhil et al., 2012).
There are a very few data in the literature on the phyosterol composition of milk thistle seed oil. The sterol pattern of
Silybium marianum has been determined in Egypt (EL-Mallah et al., 2003), Jordan (Dabbour et al., 2014) and Iran (Fathi-Achachlouei and Azadmard-Damirchi, 2009). The sterol composition can be affected by geographical growing area, difference in varieties and ripening degree of the
fruits (Casas et al., 2004; Stefanoudaki et al., 2001; Harrabi et al., 2007). No work has been published on the sterol composition during the development of milk thistle seeds. The aim of this study
was to monitor oil and sterol accumulation during seed maturation. The data obtained is important for evaluating the potential
of milk thistle seeds to be exploited as a new source of oil for nutritional, industrial and pharmaceutical applications.
2. MATERIALS AND METHODSTOP
2.1. Plant materialsTOP
Milk thistle seeds were collected from plants growing wild in Tunisia (region of Sousse), during April and June, 2012. Seeds
were picked according to external color; green seeds were chosen as immature stage, mahogany brown seeds as the intermediate
stage and dark brown seeds as the last stage of maturity (mature stage). 100 g of seeds were dried at 50 °C and then ground
to fine powder in a grinder.
2.2. Oil ExtractionTOP
The oils were extracted using petroleum ether in a Soxhlet extractor for 4 h. The solvent was initially removed using a rotary
evaporator at 40 °C. Oil samples were placed at ambient temperature (25–35 °C).
2.3. SaponificationTOP
The unsaponifiable fraction was determined by saponifying 5 g of oil extracts with 50 mL ethanolic KOH 12% (w/v) and heating
at 60 °C for 1.30 h. After cooling, 50 mL of H2O were added. The unsaponifiable matter was extracted four times with 50 mL of petroleum ether. The combined petroleum ether
extract was washed with 50 mL of ethanol–water (1:1). The extracted ether was dried over anhydrous Na2SO4 and evaporated to dryness using a rotary evaporator. The dry residue was dissolved in chloroform for TLC analysis.
2.4. Thin layer chromatographyTOP
The unsaponifiable matter was separated into sub-fractions on preparative silica gel thin-layer plates (silica gel 60G F254)
using one-dimensional TLC with hexane–diethyl ether (6:4, v/v) as the mobile solvent. The unsaponifiable fraction diluted
in chloroform was applied on the silica gel plates. After development, the plate was sprayed with 2,7-dichlorofluorescein
and viewed under UV light. The band corresponding to sterols was scraped, extracted three times with chloroform–diethyl ether
(1:1, v/v), filtered to remove the residual silica, dried in a rotary evaporator and stored at −10 °C.
2.5. Analysis of sterols by GC-MSTOP
GC-MS analyses were performed using a capillary HP-5MS column (30 m×0.25 mm I.D., 0.25 μm film thickness; Agilent Technologies)
with gas chromatography (Agilent Technologies 7820A) coupled directly to the mass detector (Agilent Technologies 5975 series
MSD). Helium was used as carrier gas, with a constant flow rate of 1 ml/ min. The injector and detector temperatures were
230 °C. The oven temperature was programmed from 150 to 320 °C at 10 °C·min−1 from 150 to 250 °C and at 5 °C·min−1 from 250 to 320 °C. Electronimpact mass spectra were measured at acceleration energy of 70 eV. Manual injection of 1 μL of
the sterol solution was performed in the split mode at a 10:1 split ratio. The phytosterol compounds were identified by comparing
their relative retention times and mass spectra with those of the authentic standard. The peaks were also confirmed by comparison
with the Wiley 275.L Mass Spectral Library.
2.6. Statistical analysisTOP
A statistical analysis was performed by using the Proc ANOVA in SAS (Software version 8). Duncan’s Multiple Range Test
was used. For each oil sample, three determinations have been made.
3. RESULTS AND DISCUSSIONTOP
3.1. Lipid contentTOP
During seed maturation the oil content increased to a maximum value of 35.8±1.3% and then declined to reach a value of 30.5±0.9%
at full maturity. More oil was synthesized during the early stage of seed development. Malekzadeh et al. (2011) reported that the total oil content of milk thistle seeds decreased under drought stress. In mature seeds, oil was stored
in the form of oil bodies (Voelker and Kinney, 2001). The extracted oil from milk thistle seed has been suggested as suitable as an edible oil (EL-Mallah et al., 2003).
3.2. Unsaponifiable fractionTOP
The results obtained showed that the amount of total unsaponifiable matter decreased during seed maturation (Table 1). The greatest change occurred during the early stage of seed development. Thus, the highest level of unsaponifiable matter
(3.8±1.2%) was detected in immature seeds. At full maturity, the unsaponifiable lipid content of the studied seeds was 1.9±0.2%
of the total oil. The total amount of unsaponifiable matter in olive oils ranged from 1 to 2% of the total lipids (Alonso-Salces
et al., 2009). Overall, the level of unsaponifiable matter ranged from 0.5 to 2.5% of the total lipids (Małecka, 2002). These minor lipids greatly influence the organoleptic quality and stability of the oil (Alonso-Salces et al., 2009). The effectiveness of unsaponifiable matter in retarding oil deterioration has been demonstrated by many researches
(Mohamed and Awatif, 1998; Gopala Krishna et al., 2003).
Table 1. Total oil and unsaponifiable matter contents of milk thistle seeds collected at three maturity stages
| Maturation stage |
Oil content (% of DW)* |
Unsaponifable matter (% of oil) |
| Immature
|
8.4±2.1 |
3.8±1.2 |
| Intermediate
|
35.8±1.3 |
2.3±0.6 |
| Mature |
30.5±0.9 |
1.9±0.2 |
| *% of DW: % of dry weight. |
The unsaponifiable fraction is made up of minor constituents (sterols, triterpene alcohols, aliphatic alcohols, hydrocarbons,
etc.), which may vary both qualitatively and quantitatively depending on genetic factor, climatic conditions, extraction and
refining procedures, as well as storage conditions (Canabate-Díaz et al., 2007). 4-Desmethylsterols were isolated from the unsaponifiable matter and represented 55.2% of this fraction and about 1% of
the total oil. This high proportion shows that milk thistle seed oil is one of the richest natural products in phytosterols
versus other vegetable oils frequently used in the diet, such as olive oil (0.17%) (Weihrauch and Gardner, 1978). These results are useful for the consumer and also for the oil producer. Indeed, the unsaponifiable fraction of vegetable
oils has applications in cosmetics and pharmacology due to its biological properties.
3.3. Sterols composition in immature seedsTOP
Phytosterols are important due to their impact on health. Therefore, readily available food products have been engineered
to be enriched in phytosterols and marketed to help lower serum cholesterol and reduce the risk cardiovascular disease. 4-Desmethylsterols
are the major components of phytosterol matter in most vegetable oils (Azadmard-Damirchi and Dutta, 2006). Eight 4-desmethylsterols were detected in the oil analyzed (Figure 1). The changes in sterol composition during the development of seeds were summarized in Table 2. The sterol content of milk thistle seeds was affected by the ripening degree of the seeds. At the early stages of seed maturation,
Δ7-stigmastenol was the most abundant sterol followed by β-sitosterol. As the seed developed, the level of Δ7-stigmastenol reduced from 52.84±1.5 to 27.81±0.5% of the total sterol content. This result could be explained by the high
activities of Δ7-sterol Δ5-desaturase and Δ7-sterol reductase during the early stages of seed development. In fact these two enzymes are involved in the conversion of
Δ7-sterols to Δ5-sterols (Benveniste, 2002). The Δ5-sterols are mainly accumulated in the plasma membrane, where they are believed to regulate the membrane fluidity (Grandmougin et al., 1989). The amounts of campesterol and stigmasterol increased gradually during seed maturation. However, the level of Δ7-avenasterol decreased as the seed developed. The results obtained show that the levels of Δ7-campesterol and Δ5-avenasterol were relatively constant. The amount of cholesterol was very low in immature seeds (<2%). The rate of cholesterol
accumulation was found to be greatest at the late stage of seed maturation.
|
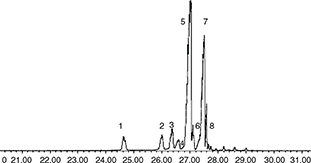 Figure 1. GC-MS Chromatogram of trimethylsilyl ether derivatives of 4-desmethyl sterols: (1) Cholesterol, (2) Campesterol, (3) Stigmasterol,
(4) Δ7-campesterol, (5) β-Sitosterol, (6) Δ5-avenasterol, (7) Δ7-Stigmastenol, (8) Δ7-Avenasterol. Figure 1. GC-MS Chromatogram of trimethylsilyl ether derivatives of 4-desmethyl sterols: (1) Cholesterol, (2) Campesterol, (3) Stigmasterol,
(4) Δ7-campesterol, (5) β-Sitosterol, (6) Δ5-avenasterol, (7) Δ7-Stigmastenol, (8) Δ7-Avenasterol.
|
|
Table 2. Evolution of sterol composition (%) in developing seeds of milk thistle
| Sterols |
Immature |
Intermediate |
Mature |
| Cholesterol |
1.10±0.2 |
1.52±0.1 |
3.91±0.3 |
| Campesterol |
1.83±0.3 |
2.30±0.5 |
4.20±0.5 |
| Stigmasterol |
1.34±0.1 |
3.60±0.3 |
5.47±0.4 |
| Δ7-Campesterol
|
2.15±0.2 |
2.27±0.1 |
2.88±0.2 |
| ß-Sitosterol |
32.20±1.3 |
37.55±1.5 |
46.50±0.8 |
| Δ5-Avenasterol |
2.24±0.4 |
3.72±0.3 |
3.20±0.1 |
| Δ7-Stigmastenol |
52.84±1.5 |
43.56±0.8 |
27.81±0.5 |
| Δ7-Avenasterol |
6.40±0.7 |
5.48±0.2 |
3.80±0.2 |
3.4. Sterol composition in mature seedsTOP
β-Sitosterol (46.50±0.8%) was the most abundant compound followed by Δ7-stigmastenol (27.81±0.5%). The β-sitosterol content determined was much higher than that in the milk thistle cultivars
grown in Iran (33–37%) (Fathi-Achachlouei and Azadmard-Damirchi, 2009), but was lower than that of the Egyptian cultivars (57.4%) (El-Mallah et al., 2003). The β-siosterol amount in milk thistle seeds is affected by environmental conditions and genotypes. The β-sitosterol
range in olive oil is 34–66% (Mezghache et al., 2010). The health aspects of β-sitosterol, the most common phytosterol, have recently been reported in several studies
(Awad et al., 1998).
The Δ7-sterol compounds were mainly represented by Δ7-stigmastenol (27.81%), Δ7-avenasterol (3.80±0.2%), and Δ7-campesterol (2.88±0.2%). The level of total Δ7-sterols (34.49%) detected in this studied oil sample was much higher than that in the Iranian samples (19–22%) (Fathi-Achachlouei
and Azadmard-Damirchi, 2009). This contrasts with the composition of corn oil where Δ7-stigmastenol amounted to 2% of the total desmehyl sterol content (Harrabi et al., 2007). Important differences may occur in some plant families. For instance, many plants belonging to the order of caryophillales
contain large amounts of Δ7- sterols; spinach and chenopodiumrubrum contain almost only Δ7-sterols such as spinasterol or stigmast-7-ene-3β-ol (Benveniste, 2002).
The campesterol (4.2±0.5%) and stigmasterol (5.47±0.4%) contents of the seed oil of this study were comparable to those of
the Iranian milk thistle cultivars (Fathi-Achachlouei and Azadmard-Damirchi, 2009), but were higher than those of olive oil where the sum of these two sterols was less than 5% (Mezghache et al., 2010). The amount of Δ5-avenasterol was 3.20±0.1%. In the literature, this component has been associated with antioxidant effects (Williamson, 1998; Blekas and Boskon, 1999). Yoshida and Niki (2003) reported that campesterol, stigmasterol and clerosterol exerted antioxidant effects on the oxidation of a methyl linoleate
oil solution. Clerosterol was not detected in the studied seed oils and in the Egyptian samples (El-Mallah et al., 2003), but was detected in the Iranian samples (Fathi-Achachlouei and Azadmard-Damirchi, 2009). Moreover, sitostanol and campestanol were absent in this studied oil sample. This result agrees with these reported by
Fathi-Achachlouei and Azadmard-Damirchi (2009) and El-Mallah et al.(2003). However, Dabbour et al. (2014), detected these two stanols in cold-pressed milk thistle seed oil (campestanol 0.21%, sitosanol 1.67%).
The results obtained show that cholesterol represents 3.91±0.3% of the total 4-desmethyl sterol fraction. It was lower than
that in the milk thistle cultivars grown in Iran (9.5%) (Fathi-Achachlouei and Azadmard-Damirchi, 2009) and in the cold-pressed seed oil of the cultivar grown in Jordan (15.14%) (Dabbour et al., 2014). Consequently, it could be suggested that the unrefined oils obtained by extraction with an organic solvent had a lower
level of cholesterol than the cold-pressed oils. Cholesterol is the predominant sterol in animal fats and fish oils, but is
very rare in vegetable oils. It is known that cholesterol occurs in the sterol fraction of many vegetable oils as a minor
component and usually amounts to 1% of the total sterol content (Phillips et al., 2002), significantly lower than that detected in the milk thistle seed oil. It has been recognized that plant sterols could reduce
plasma cholesterol levels in humans (Ostlund et al., 2002). The mechanism of cholesterol reduction in the presence of phytosterols is based on the blocked absorption of it in the
digestive tract. Since the level of cholesterol is low and in the presence of excessive amounts of phytosterols, it can be
expected that its absorption will be minimal and the positive effect of phytosterols will overcome it (Ostlund et al., 2002). Furthermore, cholesterol biosynthesis in higher plants has not been studied extensively and thus, uncertainties exist in
the sequence of intermediates. The enzymatic approach to understanding and controlling the formation of the sterol structure
was hampered by the low existence of sterol enzymes in higher plants. Therefore, milk thistle seeds which had a higher level
of cholesterol as compared with the other seeds could be a good example for the study of the cholesterol biosynthetic pathway.
4. CONCLUSIONTOP
In summary, this study provides useful information on the sterol composition in immature milk thistle seeds. Milk thistle
seeds are a rich source of phytosterols with a potential for beneficial therapeutic activities. Tunisian milk thistle oil
had a very lower amount of cholesterol as compared with the Iranian and Jordanian cultivars. The results obtained can justify
the important value of milk thistle seed oil as an attractive candidate for use in food preparation and human nutrition.
ACKNOWLEDGEMENTSTOP
The authors wish to express thanks to Mr. M. DAASSA for his technical advice.
REFERENCESTOP
| ○ |
Alonso-Salces RM, Héberger K, Holland MV, Moreno-Rojas JM, Mariani C, Bellan G, Reniero F, Guillou C. 2010. Multivariate analysis
of NMR fingerprint of the unsaponifiable fraction of virgin olive oils for authentication purposes. Food Chem. 118, 956–965. http://dx.doi.org/10.1016/j.foodchem.2008.09.061.
|
| ○ |
Awad AB, Chinnam M, Fink CS, Bradford PG. 2007. β-Sitosterol activates Fas signaling in human breast cancer cells.
Phytomedicine, 14, 747–754. http://dx.doi.org/10.1016/j.phymed.2007.01.003.
|
| ○ |
Azadmard-Damirchi S, Dutta PC. 2006. Novel solid-phase extraction method to separate 4-desmethyl, 4-monomethyl-, and 4,4-dimethylsterols
in vegetable oils. J. Chromatogr. A 1108, 183–187. http://dx.doi.org/10.1016/j.chroma.2006.01.015.
|
| ○ |
Azadmard-Damirchi S, Savage GP, Dutta PC. 2005. Sterol fractions in hazelnut and virgin olive oils and 4, 4-dimethylsterols
as possible markers for detection of adulteration of virgin olive oil. J. Am. Oil Chem. Soc. 82, 717–725. http://dx.doi.org/10.1007/s11746-005-1133-y.
|
| ○ |
Blekas G, Boskou D. 1999. Phtosterols and stability of frying oils. In: Boskou D, Elmadfa I (Eds.) Frying of Food, VA: Technomic Publishing Co., Lancaster, Pennsylvania, pp. 205–222.
|
| ○ |
Benveniste P. 2002. The Arabidopsis Book. American Society of Plant Biologists. Rockville, MD. http://www.aspb.org/publications.
|
| ○ |
Berges RR, Windeler J, Trampisch HJ, Senge T. 1995. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol
in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet, 345, 1529–32. http://dx.doi.org/10.1016/s0140-6736(95)91085-9.
|
| ○ |
Canabate-Díaz B, Segura Carretero A, Fernández-Gutiérrez A, Belmonte Vega A, Garrido Frenich A, Martínez Vidal JL, Duran Martos
J. 2007. Separation and determination of sterols in olive oil by HPLC-MS. Food Chem. 102, 593–598. http://dx.doi.org/10.1016/j.foodchem.2006.05.038.
|
| ○ |
Cercaci L, Passalacqua G, Poerio A, Rodriguez-Estrada MT, Lercker G. 2007. Composition of total sterols (4-desmethyl-sterols)
in extravirgin olive oilsobtained with different extraction technologies and their influence on the oil oxidativestability.
Food Chem. 102, 66–76. http://dx.doi.org/10.1016/j.foodchem.2006.01.062.
|
| ○ |
Dabbour IR, Al-Ismail KM, Takruri HR, Azzeh FS. 2014. Chemical Characteristics and Antioxidant Content Properties of Cold
Pressed Seed Oil of Wild Milk Thistle Plant Grown in Jordan. Pak. J. Nutr. 13, 67–78. http://dx.doi.org/10.3923/pjn.2014.67.78.
|
| ○ |
El-Mallah MH, El-Shami SM, Hassanein MM. 2003. Detailed studies on some lipids of silybummarianum (L) seed oil. Grasas Aceites, 54, 397–402.
|
| ○ |
Engelberth AS, Carrier DJ, Clausen EC. 2008. Separation of silymarins from milk thistle (Silybummarianum L.) extracted with
pressurized hot water using fast centrifugal partition chromatography. J. Liq. Chromatogr. Relat. Technol. 31, 3001–3011. http://dx.doi.org/10.1080/10826070802424907.
|
| ○ |
Fadhil AB, Ahmed KM, Dheyab MM. 2012. Silybummarianum L. seed oil: A novel feedstock for biodiesel production. Arabian J. Chem. http://dx.doi.org/10.1016/j.arabjc.2012.11.009.
|
| ○ |
Fathi-Achachlouei B, Azadmard-Damirchi S. 2009. Milk Thistle Seed Oil Constituents from Different Varieties Grown in Iran.
J. Am. Oil Chem. Soc. 86, 643–649. http://dx.doi.org/10.1007/s11746-009-1399-y.
|
| ○ |
Gopala Krishna AG, Prashanth PA, Pragasam A, Raghavendra KV, Matoon S. 2003. Unsaponifiable matter and oxidative stability
of commercially produced Indian rice bran oils. J. Food Lipids, 10, 329–340. http://dx.doi.org/10.1111/j.1745-4522.2003.tb00025.x.
|
| ○ |
Grandmougin A, Bouvier-Nave P, Ullman P, Benveniste P, Hartmann MA. 1989. Cyclopropyl sterol and phospholipids composition
of membrane fractions from maize roots treated withfenpropimorth. Plant Physiol. 90, 591–597. PMID: 16666813.
|
| ○ |
Harrabi S, Sakouhi F, St-Amand A, Boukhchina S, Kallel H, Mayer PM. 2007. Accumulation of phytoterols, triterpene alcohols
and phytostanols in developing zea mays L. kernels. J. Plant Sci. 2, 260–272. http://dx.doi.org/10.3923/jps.2007.260.272.
|
| ○ |
Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WE. 2000. Modulation of plasma lipid levels and cholesterol
kinetics by phytosterol versus phytostanol esters. J. Lipid Research, 41, 697–705. PMID: 10787430.
|
| ○ |
Karkanis A, Bilalis D, Efthimiadou A, 2011. Cultivation of milk thistle (Silybummarianum L. Gaertn.), a medicinal weed. Ind. Crops Prod. 34, 825–830. http://dx.doi.org/10.1016/j.indcrop.2011.03.027.
|
| ○ |
Malekzadeh M, Mirmazloum SI, Anguorani HR, Mortazavi SN, Panahi M. 2011. The physicochemical properties and oil constituents
of milk thistle (Silybummarianum Gaertn. cv. Budakalászi) under drought stress. J. Med. Plant. Res. 5, 1485–1488. http://www.academicjournals.org/journal/JMPR/article-full-text-pdf/B64F22017368.
|
| ○ |
Mezghache M, Henchiri C, Martine L, Berdeaux O, Aouf N, Juaneda P. 2010. Etude de la composition stérolique de trois huiles
d’olive issues des variétés Guasto, Rougette et Blanquette plantés dans l’est algérien. O.C.L. 17, 337–344. http://dx.doi.org/10.1051/ocl.2010.0330.
|
| ○ |
Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. 1995. Reduction of serum cholesterol with sitostanol-ester margarine
in a mildly hypercholesterolemic population. N. Engl. J. Med. 333, 1308–1312. http://dx.doi.org/10.1056/NEJM199511163332002.
|
| ○ |
Małecka M. 2002. Antioxidant properties of the unsaponifiable matter isolated from tomato seeds, oat grains and wheat germ
oil. Food Chem. 79, 327–330. http://dx.doi.org/10.1016/S0308-8146(02)00152-8.
|
| ○ |
Mohamed HAM, Awatif II. 1998. The use of sesame oil unsaponifiable matter as a natural antioxidant. Food Chem. 62, 269–276. http://dx.doi.org/10.1016/S0308-8146(97)00193-3.
|
| ○ |
LagardaMJ, García-Llatas G, Farré R. 2006. Analysis of phytosterols in foods. J. Pharmaceut. Biomed. Anal. 41, 1486–1496. http://dx.doi.org/10.1016/j.jpba.2006.02.052.
|
| ○ |
Li TSC, Beveridge THJ, Drover JCG. 2007. Phytosterol content of seabuckthorn (Hippophaerhamnoides L.) seed oil: Extraction
and identification. Food Chem. 101, 1633–1639. http://dx.doi.org/10.1016/j.foodchem.2006.04.033.
|
| ○ |
Ntanios F. 2001. Plant sterol-ester-enriched spreads as an example of a new functional food. Eur. J. Lipid Sci. Technol. 103, 102–106.
|
| ○ |
Ostlund RE Jr, Racette SB, Okeke A, Stenson WF. 2002. Phytosterols that are naturally present in commercial corn oil significantly
reduce cholesterol absorption in humans. Am. J. Clin. Nutr. 75, 1000–1004. PMID: 12036805.
|
| ○ |
Phillips KM, Ruggio DM, Toivo JI, Swank MA, Simpkins AH. 2002. Free and esterified sterol composition of edible oils and fats.
J. Food Compos. Anal. 15, 123–142. http://dx.doi.org/10.1006/jfca.2001.1044.
|
| ○ |
Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. 1999. Vegetables, fruits, and related nutrients
and risk of breast cancer: a case-control study in Uruguay. Nutr. Cancer, 35, 111–119. http://dx.doi.org/10.1207/S15327914NC352_3.
|
| ○ |
Sánchez Casas J, Bueno EO, García AMM, Cano MM. 2004. Sterol and erythrodiol+uvaol content of virgin olive oils from cultivars
of Extremadura (Spain). Food Chem. 87, 225–230. http://dx.doi.org/10.1016/j.foodchem.2003.11.012.
|
| ○ |
Stefanoudaki E, Chartzoulakis K, Koutsaftakis A, Kotsifaki F. 2001. Effect of drought stress on qualitative characteristics
of olive oil of cvKoroneiki. Grasas Aceites, 52, 202–206. http://dx.doi.org/10.3989/gya.2001.v52.i3-4.358.
|
| ○ |
Voelker T, Kinney AJ. 2001. Variations in the biosynthesis of seed-storage lipids. Annu. Rev. Plant Mol. Biol. 52, 335–361. PMID: 11337402.
|
| ○ |
Wallace SN, Carrier DJ, Clausen EC. 2005. Batch solvent extraction of flavonoligans from milk thistle (Silybummarianum L.
Gaertner). Phytochem. Anal. 16, 7–16. PMID: 15688950.
|
| ○ |
Weihrauch JL, Gardner JM. 1978. Sterol content of foods of plant origin. J. Am. Diet. Assoc. 73, 39–47. PMID: 659760.
|
| ○ |
Williamson E. 1998. The antioxidant activity of Δ5-avenasterol. PhD Thesis, University of Reading, Reading, UK.
|
| ○ |
Yoshida Y, Niki E. 2003. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 49, 277–280. http://doi.org/10.3177/jnsv.49.277.
|
| ○ |
Zheljazkov VD, Zhalnov I, Nedkov NK. 2006. Herbicides for weed control in blessed thistle (Silybummarianum). Weed Technol. 20, 1030–1034. http://dx.doi.org/10.1614/WT-05-135.1.
|
Figure 1. GC-MS Chromatogram of trimethylsilyl ether derivatives of 4-desmethyl sterols: (1) Cholesterol, (2) Campesterol, (3) Stigmasterol,
(4) Δ7-campesterol, (5) β-Sitosterol, (6) Δ5-avenasterol, (7) Δ7-Stigmastenol, (8) Δ7-Avenasterol.