Effect of the partial NaCl substitution by other chloride salts on the volatile profile during the ripening of dry-cured lacón
R. Domíngueza, P.E. Munekatab, A. Cittadinic and J.M. Lorenzoa,*
aCentro Tecnológico de la Carne de Galicia, Rúa Galicia N° 4, Parque Tecnológico de Galicia, San Cibrao das Viñas, 32900 Ourense, Spain
bDepartment of Food Engineering, Faculty of Animal Science and Food Engineering, University of São Paulo, 225 Duque de Caxias Norte Ave, Jardim Elite, postal code 13.635-900, Pirassununga, São Paulo, Brazil
cScuoladi Bioscienze e Medicina Veterinaria, Università di Camerino, Via Gentile III da Varano, 62032 Camerino (MC), Italy
*Corresponding author: jmlorenzo@ceteca.net
| |
SUMMARY
The influence of three salting treatments (treatment II: 50% NaCl-50% KCl; III: 45% NaCl-25% KCl-20% CaCl2-10% MgCl2; IV: 30% NaCl-50% KCl-15% CaCl2-5% MgCl2) on the formation of volatile compounds throughout the process was studied and compared to those of a control “lacón” (treatment I: 100% NaCl). There was an intense formation of volatile compounds throughout the processing, particularly during the dry-ripening
stage. The most abundant chemical family in all the formulations, in the final product was hydrocarbons followed by aldehydes.
The total volatile compound release was more intense in the control “lacóns” (1164 AU×106·g–1dry matter) than in “lacóns” from formulations II, III and IV (817–891 AU×106·g−1dry matter). The “lacóns” from formulation I showed the highest amounts of aldehydes. The “lacóns” from formulations I and
II presented the highest amounts of hydrocarbons. The main conclusion is that the replacement of NaCl produces changes in
the volatile profile and could be affect the aroma of “lacón”.
|
| |
RESUMEN
Efecto del reemplazo parcial de NaCl por otras sales en el perfil de volátiles durante la maduración del lacón crudo-curado. Se estudió la influencia de tres tratamientos de salado (tratamiento II: 50% NaCl-50% KCl; III: 45% NaCl-25% KCl-20% CaCl2-10% MgCl2; IV: 30% NaCl-50% KCl-15% CaCl2-5% MgCl2) en la formación de compuestos volátiles durante la elaboración de lacón, en comparación con un control (tratamiento I: 100%
NaCl). Hubo una intensa formación de compuestos volátiles durante el procesado, principalmente durante la fase de secado-maduración.
La familia química más abundante en el producto final fueron los hidrocarbonos, seguidos por los aldehídos. La liberación
de volátiles fue más intensa en los lacones control (1164 AU×106·g−1 materia seca) que en los otros lacones (817–891 AU×106· g−1 materia seca). Los lacones de la formulación I mostraron las mayores cantidades de aldehídos, y los lacones de las formulaciones
I y II presentaron los mayores contenidos de hidrocarburos. La principal conclusión es que el reemplazo de NaCl produce cambios
en los compuestos volátiles y por lo tanto podrían afectar al aroma del lacón.
|
1. INTRODUCTIONTOP
Dry-cured “lacón” is a traditional cured meat product made in the north-west of Spain from the fore leg of the pig which is
cut at the shoulder blade-humerus joint, following very similar manufacturing processes to those used in the production of
dry-cured ham as described by Purriños et al., (2011a). In Galicia (NW Spain), this product has been awarded Geographically Protected Identity (G.P.I.) (Official Journal of the European Communities, 2001).
Salt is an essential ingredient in dry-cured “lacón” due to its contribution to the water-holding capacity, prevention of
microbial growth, and reduction in water activity which facilitate the solubilization of certain proteins and confer a typical
salty taste (Lorenzo et al., 2008; Purriños et al., 2011b; Purriños et al., 2013). Moreover, the salt affects some chemical and biochemical reactions such as proteolysis, lipolysis and lipid oxidation,
which contribute to the development of texture and typical flavor of dry-cured “lacón” (Garrido et al., 2012; Purriños et al., 2012).
In recent years different studies have begun to show that meat consumption is being more and more influenced by health and
nutritional considerations. The mean daily sodium intake of the European population ranges from about 3 to 5 g (8 to 11 g
NaCl) (EFSA, 2005). On a general basis, it has been established that the consumption of more than 6 g NaCl/day/person is associated with aging
and increase in blood pressure. Therefore, limiting dietary sodium intake should be achieved by restricting daily salt (sodium
chloride) consumption to less than 5 g per day (WHO/FAO, 2003). In the case of Spain, in 2008 the Spanish Food Safety and Nutrition Agency started a salt reduction plan with certain specific
goals which would enable its intake to go down from the current value of 9.7 g/day to an intake of less than 8.0 g/day by
2014.
Due to the increased knowledge about the links between sodium intake and coronary heart diseases, consumers’ demand for low-salt
meat products with the same quality as conventional ones has increased. To this regards, the partial substitution of NaCl
by a mixture of salts (potassium, calcium, and magnesium salts) appears to be the best alternative to reduce the sodium content
in dry-cured “lacón” (Lorenzo et al., 2015). However, the effect of the partial NaCl replacement by other chloride salts on the volatile profile of dry-cured “lacón”
is unknown.
The aim of this work was to determine the influence of partial NaCl replacement by a mixture of KCl, CaCl2 and MgCl2 on the generation and release of volatile compounds to the headspace of dry-cured “lacón” for their possible contribution to the flavor of this product.
2. MATERIAL AND METHODSTOP
2.1. SamplesTOP
Fifty-two fresh “lacón” pieces with an average weight of 4.61±0.47 kg each were obtained from a local slaughterhouse in the
area of Ourense (Spain). Four of the raw pieces were sampled and analyzed in order to characterize the raw material. The remaining
forty-eight “lacón”raw pieces were submitted to the traditional “lacón” processing (Purriños et al., 2011a). The “lacón” samples were randomly divided into four batches with twelve “lacóns” in each batch. “Lacóns” from the first
batch were salted with the traditional NaCl (100% NaCl, treatment I) and were used as the control of the volatile compounds,
whereas the other batches were salted in the same way but with partial replacements of NaCl by other salts. So, the second
batch was salted with 50% NaCl and 50% KCl (treatment II); the third batch with 45% NaCl, 25% KCl, 20% CaCl2 and 10% MgCl2 (treatment III); and the fourth batch with 30% NaCl, 50% KCl, 15% CaCl2 and 5% MgCl2 (treatment IV). The salting stage was carried out between 2 and 5 °C and the relative humidity (HR) between 80 and 90% for
a total of 5 days. After the salting stage the pieces were taken from the heap, brushed, washed, and transferred to a post-salting
room where they stayed for 14 days at 2–5 °C and around 85–90% HR. After the post-salting stage the pieces were transferred
to a room at 12 °C and 74–78% HR where a drying-ripening process took place for 84 days. Samples were taken after the salting,
post-salting, and drying-ripening stages. In each sampling point, a total of 4 “lacón” samples from each batch were analyzed.
The “Lacón” pieces were skinned, deboned, and the triceps brachii muscle was extracted. The samples were vacuum packed and stored at −30 °C for no longer than four weeks until analysis.
2.2. Chemical composition and pH valuesTOP
Moisture, fat and protein contents were determined according to the Association of Official Analytical Chemists (2005) in triplicate. The moisture content was determined by drying in an oven at 105 °C±2 °C; the nitrogen content was determined
by the Kjeldahl method and the protein content was estimated by multiplying the nitrogen content by 6.25; the fat content
was determined by the Soxhlet method using petroleum ether as solvent. The pH of the samples was measured using a digital
pH meter (model 710 A+, Thermo Orion, Cambridgeshire, UK) equipped with a penetration probe.
2.3. Mineral compositionTOP
The quantification of mineral elements (Na, K, Ca and Mg) was performed by inductively coupled plasma-optical emission spectroscopy
(ICP-OES), according to the procedure described by Lorenzo et al. (2015). The final value for each element was obtained by calculating the average of three determinations.
2.4. Volatile compound profileTOP
The extraction of the volatile compounds was performed using solid-phase micro-extraction (SPME). A SPME device (Supelco,
Bellefonte, PA, USA) containing a fused-silica fiber (10 mm length) coated with a 50/30 μm thickness of DVB/CAR/PDMS (divinylbenzene/carboxen/polydimethylsiloxane)
was used for HS-SPME extraction.
The muscle samples were ground with a commercial grinder, a 1 g portion was weighed into a 24 mL vial and the vial was screw-capped
with a laminated Teflon rubber disk. The fiber was inserted into the sample vial through the septum and then exposed to the
headspace. The extractions were carried out in an oven to ensure a homogeneous temperature for sample and headspace. The fiber
was conditioned prior to analysis by heating it in a gas chromatograph injection port at 270 °C for 60 min, following the
manufacturer specifications. Extraction was performed at 35 °C for 30 min. Before extraction, the samples were equilibrated
for 15 min at the temperature used for extraction. Once sampling was finished, the fiber was drawn into a needle and transferred
to the injection port of the gas chromatograph–mass spectrometer (GC–MS) system.
A gas chromatograph 6890N (Agilent Technologies Spain, S.L., Madrid, Spain) equipped with a mass detector 5973N (Agilent Technologies
Spain, S.L., Madrid, Spain) was used with a DB-624 capillary column (J&W scientific: 30 m, 0.25 mm id, 1.4 μm film thickness).
The SPME fiber was desorbed and maintained in the injection port at 260 °C for 8 min. The sample was injected in the splitless
mode. Helium was used as carrier gas with a linear velocity of 40 cm·s−1. The temperature programme was isothermal for 10 min at 40 °C, raised to 200 °C at a rate of 5 °C·min−1, and then raised to 250 °C at a rate of 20 °C·min−1, and held for 5 min: total run time 49.5 min. Injector and detector temperatures were both set at 260 °C. The mass spectra
was obtained using a mass selective detector working in electronic impact at 70 eV, with a multiplier voltage of 1953 V and
collecting data at a rate of 6.34 scans·s−1 over the range m/z 40-300.
The compounds were identified comparing their mass spectra with those contained in the NIST05 (National Institute of Standards
and Technology, Gaithersburg) library, and/or by comparing their mass spectra and retention time with standards (Supelco,
Bellefonte, PA, USA), and/or by calculation of retention index relative to a series of standard alkanes (C5–C14) (for calculating linear retention index, Supelco 44585-U, Bellefonte, PA, USA) and matching them with data reported in the
literature. The results are expressed as AU (area units)×106·g−1 of dry matter.
2.5. Statistical analysisTOP
All statistical analyses were performed using the IBM SPSS Statistics 19 software (IBM, Corp, 2010). The normality was assessed using the Kolmogorov-Smirnov test, and the Levene's homogeneity of variance test was applied
to examine the equality of variances. After verification of normal distribution and constant variance of data, significant
differences were determined using one-way analysis of variance (ANOVA). A Duncan's test was performed to compare the mean
values for processing time (0, 5, 19 and 103 days) and partial sodium replacement at a significance level of P<0.05.
3. RESULTS AND DISCUSSIONTOP
3.1. Effect of the salting treatments on physicochemical properties and mineral contentTOP
The results of chemical composition and pH values at the end of dry-cured “lacón” submitted to four different salting treatments
are shown in Table 1. Moisture content was significantly (P<0.05) affected by NaCl replacement by other salts, since “lacóns” submitted to formulations IV presented the highest values.
These differences could be due to the quicker penetration of the salt mixtures containing KCl that would hinder the exit of
water from the inside of the meat (Aliño et al., 2009). This finding is in agreement with those reported by Wu et al. (2014) who observed significantly (P<0.05) higher moisture contents in bacon samples salted with 30% NaCl and 70% KCl compared with samples salted with 100% NaCl.
However, Armenteros et al. (2012a) did not find significant differences among salting formulations on the moisture content of dry-cured ham. On the other hand,
the protein and intramuscular fat contents did not show significant differences among treatments.
Table 1. Chemical composition and pH values of dry-cured lacón at the end of processing (mean ± standard deviation of four
replicates)
|
Salt formulations |
SEM |
Sign. |
| I |
II |
III |
IV |
| Moisture (%) |
55.28±3.83ab |
53.21±2.92a |
58.87±0.87bc |
61.66±0.28c |
1.12 |
* |
| Protein (% of dry matter) |
33.28±2.77 |
36.76±4.07 |
31.03±2.91 |
32.09±3.66 |
0.94 |
n.s. |
| Fat (% of dry matter) |
9.82±2.52 |
7.65±2.55 |
6.19±1.57 |
7.50±2.85 |
0.63 |
n.s. |
| pH |
5.96±0.08ab |
6.08±0.09b |
5.83±0.05a |
5.92±0.05a |
0.03 |
** |
| Salt formulations: treatment I: control, 100% NaCl; treatment II: 50% NaCl and 50% KCl; treatment III: 45% NaCl, 25% KCl, 20% CaCl2 and 10% MgCl2; treatment IV: 30% NaCl, 50% KCl, 15% CaCl2 and 5% MgCl2 |
| a–c Mean values in the same row (corresponding to the same parameter) not followed by a common letter differ significantly (P<0.05).
|
| Sign.: Significance; n.s.: not significant; * (P<0.05); *** (P<0.001).
|
Regarding pH values, the NaCl replacement by other salts induced significant (P<0.01) differences among treatments. In this study the “lacóns” salted with lower NaCl concentrations (formulations III and
IV) presented the lowest values of pH, while the highest values were obtained in “lacóns” from formulation II. Conflicting
data are available in the literature about KCl, MgCl2, and CaCl2 inclusion in dry-cured meat products regarding this aspect. To this regards, Gimeno et al. (1999) studied the effect of a mixture of NaCl (10 g·kg−1), KCl (5.52 g·kg−1), MgCl2 (2.35 g·kg−1), and CaCl2 (4.64 g·kg−1) to partially substitute NaCl and reported a greater pH decrease in the reduced NaCl formulation compared to the traditional one. However, Zanardi et al., (2010) noticed that the salt mixture (NaCl 13.5 g·kg−1, KCl 4.2 g·kg−1, CaCl2 2.4 g·kg−1, and MgCl2 2.4 g·kg−1) did not affect the pH evolution throughout the processing.
Table 2 shows the mineral content of dry-cured “lacón” submitted to four different salting treatments. With regards to the natural
content of minerals in the “lacón”, the salt composition at the end of the ripening period inside the “lacón” is somehow reflected
in the salt penetration and diffusion through the “lacón”. A significant reduction (P<0.001) in the Na content was achieved through the partial substitution of NaCl by the mixture of chloride salts employed
during their production. In our study, the “lacón” samples submitted to formulation I presented the highest amount of sodium
(2446.6 mg·100 g−1), which was similar to a normal level for “lacón” with these characteristics (Lorenzo et al., (2003). The partial substitution of sodium chloride by the mixture of chloride salts significantly (P<0.001) reduced the sodium content and increased potassium, calcium and magnesium contents. However, lower concentrations
of Ca+2 and Mg+2 were found in the “lacón” in relation to the proportions of these chloride salts employed during their production. These
findings are in agreement with those obtained by Aliño et al. (2009) and Armenteros et al. (2009) who also observed the difficulty of divalent cations to penetrate inside the muscle. This could be explained by the fact
that Ca+2 and Mg+2 cations have higher charge density (0.050 and 0.082 units of charge/molecular weight, respectively) that would increase their
difficulty to penetrate inside the “lacón” (Blesa et al., 2008).
Table 2. Mineral composition (ppm) of dry-cured lacón at the end of processing (mean ± standard deviation of four replicates)
| ppm |
Salt formulations |
SEM |
Sign. |
| I |
II |
III |
IV |
| Na |
2446.60±108.46c |
1483.91±118.55b |
738.21±20.34a |
586.35±109.39a |
189.50 |
*** |
| K |
565.11±22.22a |
1915.42±78.12b |
876.08±80.46b |
1668.94±99.21c |
127.61 |
*** |
| Ca |
9.35±1.27a |
9.31±0.59a |
42.87±10.48b |
33.73±11.08b |
4.19 |
*** |
| Mg |
35.04±5.35a |
31.89±3.35a |
42.73±2.14b |
36.97±3.70b |
1.64 |
* |
| Salt formulations: treatment I: control, 100% NaCl; treatment II: 50% NaCl and 50% KCl; treatment III: 45% NaCl, 25% KCl, 20% CaCl2 and 10% MgCl2; treatment IV: 30% NaCl, 50% KCl, 15% CaCl2 and 5% MgCl2 |
| a–d Mean values in the same row (corresponding to the same parameter) not followed by a common letter differ significantly (P<0.05).
|
| Sign.: significance: n.s.: not significant; * (P<0.05); ***(P<0.01).
|
3.2. Effect of the salting treatments on volatile compoundsTOP
The SPME technique is not normally used for absolute quantifications, but when exactly the same extraction methodology is
applied, this technique allows for comparing relative amounts among samples. A total of 31 volatile compounds were detected
in the headspace of “lacón” at the end of the dry-ripening process. The compounds were grouped by chemicals and their linear
retention index (Table 3), comprising 3 acids, 4 alcohols, 4 aldehydes, 2 esters, 15 hydrocarbons, 2 ketones and 1 furan. At the end of the process
the volatile compound profile maintained the relationship hydrocarbons>aldehydes>alcohols>acids>ketones>furan>esters. Lorenzo et al. (2014) also reported that hydrocarbons were the most abundant compounds in a previous study of dry-cured “lacón”. However, other
studies found that the most abundant group of volatile compounds were alcohols (Wu et al., 2015), esters (Bermúdez et al., 2015; Gómez and Lorenzo, 2013; Lorenzo, 2014; Lorenzo and Fonseca, 2014) or aldehydes (Armenteros et al., 2012b; Purriños et al., 2011b; Purriños et al., 2012). These different results could be explained by the volatile extraction methods used, since the purge and trap extraction
and the SPME technique offer potentially different profiles of volatile compounds (Bermúdez et al., 2015). In addition, there are many factors that affect SPME fiber performance, such as the choice of stationary phase and the
extraction conditions.
Table 3. Volatile compounds (AU×106·g−1 dry matter) in the headspace of dry-cured “lacón” salted with different salt formulations at the end of processing. Data are
the average values of four replicates
|
|
|
Salt formulations |
SEM |
Sign. |
| LRI |
R |
I |
II |
III |
IV |
| Acetic acid |
720 |
ms,lri |
2.47 |
1.81 |
1.88 |
1.31 |
0.37 |
ns |
| Butanoic acid |
883 |
ms,lri |
15.58b |
20.92b |
22.53b |
5.16a |
2.94 |
*** |
| Nonanoic acid |
1370 |
ms,lri |
0.57b |
0.00a |
0.67b |
0.00a |
0.10 |
*** |
| Total acids |
|
|
19.64b |
22.01b |
24.87b |
5.78a |
3.18 |
* |
| |
|
|
|
|
|
|
|
|
| 1-Pentanol |
834 |
ms, lri |
21.35b |
5.59a |
14.83b |
23.77b |
2.71 |
* |
| 1-Hexanol |
925 |
ms, lri |
7.27b |
0.00a |
20.89c |
16.63c |
2.59 |
*** |
| 1-Octen-3-ol |
1095 |
ms, lri |
24.32ab |
16.61a |
32.84bc |
41.94c |
3.36 |
** |
| Benzyl alcohol |
1107 |
ms,lri |
15.84 |
12.55 |
13.29 |
11.66 |
0.85 |
ns |
| Total alcohols |
|
|
64.80b |
35.82a |
63.94b |
93.99c |
6.44 |
*** |
| |
|
|
|
|
|
|
|
|
| Pentanal |
736 |
ms,lri, s |
15.64a |
0.00b |
13.84a |
12.05a |
2.34 |
*** |
| Hexanal |
823 |
ms,lri, s |
608.55c |
40.97a |
297.10b |
274.90b |
59.87 |
*** |
| Heptanal |
933 |
ms,lri, s |
8.32ab |
6.46a |
12.31b |
16.20c |
1.29 |
** |
| Decanal |
1347 |
ms,lri, s |
3.92c |
2.60b |
0.00a |
0.00a |
0.53 |
*** |
| Total aldehydes |
|
|
626.52c |
49.38a |
318.64b |
299.13b |
61.48 |
*** |
| |
|
|
|
|
|
|
|
|
| Ethanol, 2-(2-butoxyethoxy)-, acetate |
1419 |
ms,lri |
1.69b |
1.27b |
0.00a |
0.00a |
0.25 |
** |
| 1,2,3-Propanetriol, diacetate |
1514 |
ms |
1.10b |
0.87b |
0.00a |
0.00a |
0.17 |
** |
| Total esters |
|
|
2.79b |
1.85b |
0.00a |
0.00a |
0.39 |
*** |
| |
|
|
|
|
|
|
|
|
| Pentane, 2,3,4-trimethyl- |
660 |
ms |
3.81 |
5.01 |
2.66 |
2.07 |
0.51 |
ns |
| Heptane |
700 |
ms,lri, s |
2.71 |
2.26 |
2.40 |
0.63 |
0.39 |
ns |
| Pentane, 2,3,3-trimethyl- |
706 |
ms,lri |
12.23b |
9.38b |
3.24a |
3.99a |
1.25 |
** |
| Octane |
800 |
ms,lri, s |
14.1 |
27.82 |
14.79 |
16.56 |
2.45 |
ns |
| Heptane, 3-methylene- |
807 |
ms,lri |
2.63b |
3.42b |
0.00a |
0.00a |
0.52 |
*** |
| Hexane, 3-ethyl- |
860 |
ms |
1.30ab |
1.82b |
0.00a |
1.93b |
0.32 |
ns |
| Heptane, 2,2,4-trimethyl- |
904 |
ms,lri |
7.29b |
7.37b |
0.00a |
0.00a |
1.20 |
*** |
| Heptane, 2,5,5-trimethyl- |
924 |
ms,lri |
5.42b |
6.73b |
0.00a |
0.00a |
0.89 |
*** |
| Heptane, 2,2,4,6,6-pentamethyl- |
998 |
ms,lri |
569.66b |
627.46b |
376.47a |
428.29a |
37.99 |
* |
| 3-Ethyl-3-methylheptane |
1003 |
ms |
1.73b |
2.14b |
1.64b |
0.00a |
0.27 |
** |
| Decane, 2,6,7-trimethyl- |
1134 |
ms |
48.14b |
30.57ab |
37.37b |
0.00a |
7.54 |
* |
| Dodecane |
1200 |
ms,lri, s |
3.97 |
3.51 |
2.68 |
2.45 |
0.28 |
ns |
| Undecane, 5-methyl- |
1207 |
ms,lri |
5.63c |
4.77bc |
4.01ab |
3.00a |
0.40 |
* |
| Decane, 5-methyl-6-methylene- |
1265 |
ms,lri |
3.16 |
3.95 |
3.08 |
1.98 |
0.46 |
ns |
| Tridecane |
1300 |
ms,lri, s |
1.39 |
1.42 |
2.04 |
1.83 |
0.13 |
ns |
| Total hydrocarbons |
|
|
650.12b |
698.82b |
432.59a |
459.53a |
40.89 |
* |
| |
|
|
|
|
|
|
|
|
| 2-Butanone, 3-hydroxy- |
765 |
ms,lri |
15.87c |
7.76ab |
4.02a |
11.86b |
1.52 |
* |
| 2-Heptanone |
940 |
ms,lri |
3.73a |
2.73a |
5.61b |
3.49a |
0.33 |
** |
| Total ketones |
|
|
20.43c |
10.95ab |
6.42a |
15.35b |
1.74 |
** |
| |
|
|
|
|
|
|
|
|
| Furan, 2-pentyl- |
1009 |
ms,lri |
4.06 |
3.80 |
6.05 |
5.80 |
0.53 |
ns |
| |
|
|
|
|
|
|
|
|
| Total compounds |
|
|
1164.38b |
817.24a |
873.03a |
891.36a |
53.31 |
* |
| Salt formulations: I: (100% NaCl), II: (50% NaCl and 50% KCl), III: (45% NaCl, 25% KCl, 20% CaCl2 and 10% MgCl2), IV: (30% NaCl, 50% KCl, 15% CaCl2 and 5% MgCl2). Sign.: significance; ns: not significant; *P<0.05; **P<0.01; ***P<0.001. a-c Means in the same row not followed by a common superscript letter differ significantly (P<0.05; Duncan test); S.E.M.: Standard error of the mean; AU: area units resulting of counting the total ion chromatogram (TIC) for each compound;
LRI: linear retention index calculated for DB-624 capillary column (J&W scientific: 30 m×0.25 mm id, 1.4 μm film thickness)
installed on a gas chromatograph equipped with a mass selective detector; R: Reliability of identification; LRI: volatiles
identified by comparing their LRI with those reported in the literature (Lorenzo and Fonseca, 2014; Lorenzo and Domínguez, 2014; Domínguez et al., 2014); ms: mass spectrum agreed with mass database (NIST05); s: mass spectrum and retention time identical to an authentic standard.
|
The changes in the most relevant volatile compounds during “lacón” processing are shown in Figure 1. The production and release of the volatile compounds increased throughout the dry-cured “lacón” processing from 82 AU×106·g−1 dry matter in fresh meat to 1164, 817, 873 and 891 AU×106 ·g−1 DM at the end of dry-ripening of “lacóns” from formulations I, II, III and IV, respectively. Our results are in agreement
with other studies with different meat products, such as “cecina” (Lorenzo, 2014), “lacón”(Lorenzo and Fonseca, 2014; Purriños et al., 2012), dry-cured ham (Armenteros et al., 2012b; Bermúdez et al., 2015), dry-cured loin (Lorenzo and Carballo, 2015) and bacon (Wu et al., 2015). The production of volatile compounds was particularly high during the dry-ripening stage. This fact could be due to the
increase in temperature and reduction in moisture in the course of the process (Lorenzo and Fonseca, 2014).
|
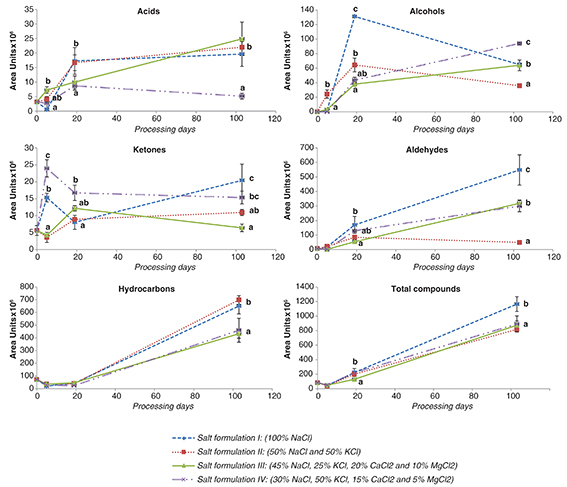 Figure 1. Evolution of volatile compounds throughtout dry-cured “lacón” processing using different salting formulations. Error bars
indicate the standard error for each treatment. Different letters indicate significant differences among formulations (P<0.05). Plotted values are the means of four replicates. Figure 1. Evolution of volatile compounds throughtout dry-cured “lacón” processing using different salting formulations. Error bars
indicate the standard error for each treatment. Different letters indicate significant differences among formulations (P<0.05). Plotted values are the means of four replicates.
|
|
As mentioned above, hydrocarbons were the most abundant chemical group at the end of the ripening process in dry-cured “lacón”.
They represent 55, 49 and 51% of the total volatile compounds in “lacóns” from formulations I, III and IV, respectively, and
85% of the total volatile compounds in “lacóns” from formulation II. In this study, heptane, 2,2,4,6,6-pentamethyl, was the
most abundant hydrocarbon in all the batches (representing about 90% of total hydrocarbons), followed by decane, 2,6,7-trimethyl
and octane. These results agree with Bermúdez et al. (2015) who reported that the heptane, 2,2,4,6,6-pentamethyl was the most important aliphatic hydrocarbons in dry-cured hams. Aliphatic
hydrocarbons with less than ten carbons atom arise mainly from lipid oxidation (Ruíz et al., 2002), while those with longer chains could be accumulated in the fat depots of the animal, probably from feeding.
An increase in the total amount of hydrocarbons was observed during the process, from initial values of 72 AU×106·g−1 DM in fresh meat to 650, 698, 432 and 459 AU×106 ·g−1 DM at the end of the dry-ripening of samples from formulations I, II, III and IV, respectively (Figure 1). In the salting and post-salting stages, the contents of hydrocarbons decreased and subsequently increased. This tendency
was previously described in “lacón” (Purriños et al., 2012). On the contrary, Armenteros et al. (2012b) reported that hydrocarbons increased during the post-salting stage and decreased in the final stage of the process. In this
study, the production of hydrocarbons was not affected during the salting and post-salting stages by the partial replacement
of NaCl content by other chloride salts, but their amounts increased at different rates up to the end of the dry-ripening
stage. Furthermore, the total content of hydrocarbons was significantly higher (P<0.05) in cured “lacóns” from formulations I and II than those from III and IV. This fact was mainly due to the significantly
higher values (P<0.05) of heptane, 2,2,4,6,6-pentamethyl, but also due to higher values (P<0.001) of pentane, 2,3,3-trimethyl, heptane, 3-methylene, heptane, 2,2,4-trimethyl and heptane, 2,2,5-trimethyl in formulations
I and II than in the other ones. In this case, NaCl could have contributed to an increased release of these compounds. On
the contrary, Armenteros et al. (2012b) found higher quantities of branched hydrocarbons in hams with the replacement of NaCl than in the control batch (salted with
100% NaCl). Despite the high content of these compounds, they probably had a low impact on flavor due to their relatively
high odor thresholds (Domínguez and Lorenzo, 2014).
In the present study, aldehydes represented about 35–50% of the total volatile compounds in the “lacóns” from batches I, III
and IV and 5.9% of the total volatile compounds in “lacóns” from batch III. Regarding the aldehydes, hexanal, derived from
the n-6 fatty acids such as linoleic and arachidonic acids was the most abundant (Węsierska et al., 2013). It represented between 80% and 95% of total aldehydes. These results agree with those reported by Lorenzo et al. (2014) and Lorenzo and Fonseca (2014) who observed that hexanal was the most abundant aldehyde in dry cured “lacón” and it was considered a good indicator of the
lipid oxidation in this meat product. Moreover, hexanal was also the most important aldehyde in dry-cured bacon (Wu et al., 2015), “cecina” (Lorenzo, 2014), ham (Armenteros et al., 2012b; Bermúdez et al., 2015) and dry-ripened “chorizo” (Gómez and Lorenzo, 2013). The high levels of straight chain aldehydes, such as pentanal, hexanal, heptanal and decanal, indicated that lipid oxidation
occurred actively during the dry-curing process and played a very important role in the formation of headspace volatiles by
cured meat products (Wu et al., 2015).
The amount of aldehydes increased from initial values of 6.45 AU×106·g−1 DM in the raw pieces to 626, 318 and 299 AU×106·g−1 DM at the end of dry-ripening of “lacóns” submitted to formulations I, III and IV, respectively. These results were previously
reported by Armenteros et al. (2012b) and Lorenzo and Carballo (2015) who noticed that total aldehydes increased during the process of dry-cured meat products. However, in “lacóns” from formulation
II, the values of aldehydes increased until the end of the post-salting stage (85.15 AU×106·g−1 DM) and then decreased in the final stage (49.38 AU×106·g−1 DM). These findings are in agreement with those reported by Lorenzo et al. (2014), Lorenzo (2014) and Lorenzo and Fonseca (2014) who noticed a decreased during the final stages. The decrease in aldehydes in “lacóns” submitted to formulation II at the
end of the process could be the reflection of their participation in other chemical reactions yielding other volatile or non-volatile
compounds (Armenteros et al., 2012b).
On the other hand, hexanal showed differences (P<0.001) among the batches. The “lacóns” from formulation I had the highest values (608.55 AU×106·g−1 DM) and the lowest were presented by the “lacóns” submitted to formulation II (40.97 AU×106·g−1 DM). The other 2 batches presented intermediated values (297.10 and 274.90 AU×106·g−1 DM for the “lacóns” from formulations III and IV, respectively). Pentanal was found in the samples submitted to formulations
I, III and IV (values between 12.05 and 15.64 AU×106·g−1 DM), while the highest values of heptanal were observed in the “lacóns” submitted to formulation IV (16.20 AU×106·g−1 DM) in comparison with the other batches (between 6.46 and 12.31 AU×106·g−1 DM). The formulations I and II also presented low values of decanal.
Armenteros et al. (2012b) and Purriños et al. (2012) also found that dry-cured products with high contents of NaCl showed the highest amounts of hexanal. According to these authors,
the higher values of hexanal in “lacóns” submitted to formulation I might be related with the fact that NaCl could have exerted
a pro-oxidant effect. This result was also reported by Du and Ahn (2005) who noticed that NaCl (less than 2%) was a pro-oxidant in meat and meat products. Another possible explanation could be
that the replacement of NaCl by other salts affects the degree of proteolysis in ripened meats as NaCl inhibits muscle proteases
(Garrido et al., 2012), and a significant reduction in such ions in the meat systems generally leads to a more intense proteolysis (Estévez, 2011). A large production of free amino acids and peptides in “lacóns” from formulations II, III and IV could have contributed
to inhibiting lipid oxidation and, hence, the formation of lipid derived volatiles such aldehydes. On the contrary, Andrés et al. (2007) did not observe variations in the hexanal contents among dry-cured hams with different salt contents. Aldehydes are known
as the major contributors to the unique flavor of dry-cured products due to their low odor thresholds (Bermúdez et al., 2015).
Alcohols were the third chemical family after the ripening period, they represented between 4 and 10% of the total volatile
compounds, and four different alcohols were identified: 1-pentanol, 1-hexanol, 1-octen-3-ol and benzyl alcohol. These compounds
have also been detected in other dry-cured meat products (Bermúdez et al., 2015; Lorenzo et al., 2014; Purriños et al., 2012). Among the alcohols, in all the batches, 1-octen-3-ol was the most abundant and represented about 40% of the total alcohols.
Our results agree with those reported by Bermúdez et al. (2015), Lorenzo et al. 2014 and Lorenzo and Fonseca (2014), who found that the most abundant alcohol at the end of the dry-ripened stage was 1-octen-3-ol.
The formation and release of alcohols was affected from the beginning by the processing time, as well as by the formulation.
In fresh meat alcohols were not detected, and they increased during cold stages. After the post-salting stage the values of
alcohols were 131.2, 64.4, 37.9 and 43.4 AU×106·g−1 DM in “lacóns” from formulation I, II, III and IV, respectively. Then, total alcohols of the samples from formulations I
and II gradually declined to the end of the process, while in samples submitted to formulations III and IV increased to the
end of the process (Figure 1). The amount of total alcohols was significantly (P<0.05) higher in “lacóns” from formulation IV (93.9 AU×106·g−1 DM), followed by “lacóns” submitted to formulations I and III (64.8 and 63.9 AU×106·g−1 DM) and finally the “lacóns” from formulation II (35.8 AU×106·g−1 DM).
The samples submitted to formulation IV presented the higher values of 1-octen-3-ol, 1-pentanol and 1-hexanol than the samples
from formulations I and II. The intermediate values were observed in samples from formulation III. Our results are in agreement
with Armenteros et al. (2012b), who found the highest amounts in the samples with replacement of NaCl. This result probably was due to a more intense chemical
reduction of corresponding aldehydes by microbial enzymes in “lacóns” submitted to treatment IV. NaCl is known to have antimicrobial
activity, and a partial replacement could explain a more intense microbial growth and a higher production of these compounds.
It is in agreement with reported by Lorenzo et al. (2015) in a previous study, who found that lacóns from formulations III and IV presented the highest counts of total viable counts,
salt tolerant flora and yeasts. In contrast, Purriños et al. (2012) found that the “lacóns” with high salt content had higher amount of alcohols than in “lacóns” with reduced salt content.
Only three acids were detected through the manufacture process of dry-cured “lacón”. Butanoic acid was the most abundant at
the end of the process representing about 80–90% of total acids. These findings are in agreement with the study of Bermúdez et al. (2015) in dry-cured hams. However, other authors found that acetic acid was the main acid detected in dry-cured products (Lorenzo et al., 2014; Wu et al., 2015). During cold stages, the generation of acids was affected by partial replacement of NaCl by other salts. After post-salting
stage, the batches I and II presented higher (P<0.05) values of acids (17.3 and 16.7 AU×106·g−1 DM, respectively) than batches III and IV (9.9 and 8.7 AU×106·g−1 DM, respectively). Then, the evolution of acids was different depending on the batches; in the samples submitted to formulations
I, II and III the acid content increased to the final of dry-ripened process. However, the acid content of the “lacóns” from
batch II decreased at the end of the process (Figure 1) and is in agreement with those reported by Lorenzo and Fonseca (2014) who also found that the acids content decreased after post-salting stage. At the end of the final stage, were not found differences
among samples submitted to formulations I, II and III (19.6, 22.0 and 24.9 AU×106·g−1 DM, respectively), while the samples from batch IV showed significantly (P<0.05) lower values (5.78 AU×106·g−1 DM) than the other batches. These differences were mainly related to the differences in butanoic acid content (15.6, 20.9,
22.5 and 5.78 AU×106·g−1 DM for samples from formulations I, II, III and IV, respectively).
In this study, two ketones were detected in the headspace of “lacón”. The most abundant ketone was 2-butanone, 3-hydroxy representing
about 70–75% of total ketones. In contrast, Bermúdez et al. (2015) and Lorenzo and Fonseca (2014) reported that 2-heptanone was the most abundant ketone in dry-cured ham and dry-cured “lacón”, respectively, while Wu et al. (2015) noticed that 2-butanone and 2-butanedione were the predominant ketones in bacon. At the end of the processing, “lacóns” submitted
to formulation I had significantly (P<0.05) higher levels of ketones than those from the other formulations. “Lacóns” submitted to formulation III presented the
highest values of 2-heptanone (5.61 AU×106·g−1 DM), while the values of 2-butanone, 3-hydroxy were significantly (P<0.05) higher in the “lacóns” from treatment I than from the other formulations. Our results are in agreement with those reported
by Armenteros et al. (2012b), who found the highest values of 2-butanone, 3-hydroxy in control dry-cured hams (salted with 100% NaCl) than in dry-cured
hams with salt replacement.
Only furan, 2-pentyl, was detected after the dry-ripening process (values between 3.8 and 6.1 AU× 106·g−1 DM) and it did not show differences among treatments. Furan, 2-pentyl, has been found in other dry-cured meat products manufactured
from whole pieces (Bermúdez et al., 2015; Lorenzo et al., 2014; Lorenzo and Carballo, 2015). This furan is a non-carboxylic compound derived from linoleic acid and other n-6 fatty acids, with a relatively low threshold and vegetable aromatic note (Fay and Brevard, 2005).
Finally, esters were only detected in samples from formulations I and II, but their amounts were very low (2.79 and 1.85 AU×106·g−1 DM in the “lacóns” submitted to formulations I and II, respectively), and they represented less than 0.02% of the total volatile
compounds. Esters have low olfaction threshold values. However, taking into account that the samples have very low values
of these compounds, it can be considered that they do not contribute to the aroma of “lacón”.
In addition, in a previous study (Lorenzo et al., 2015), the sensory analysis showed that the panellists gave the highest scores of intensity odor to “lacóns” salted with 100%
NaCl (formulation I) and the lowest values were observed in “lacóns” salted with 50% NaCl and 50% KCl (formulation II). As
discussed above, the odor of “lacóns” not only depends on the amount of volatile compounds, but also for their threshold.
However, the “lacóns” from formulation I, which had the highest amount of total volatile compounds also presented the highest
scores of odor intensity according to Lorenzo et al. (2015), while the “lacóns” from formulation II had the lowest values of total volatile compounds and odor intensity (Lorenzo et al., 2015). Therefore, in this case it seems that the amount of total volatile compounds was directly related to odor intensity.
4. CONCLUSIONSTOP
The formation of the volatile compounds significantly increased during the dry-curing process, particularly during the dry-ripening
stage. The replacement of NaCl by other salts influenced the formation of the majority of volatile compounds. At the end of
processing, the control samples (salted with 100% of NaCl) showed the highest values of total volatile compounds. This fact
is mainly due to higher values of hexanal and total aldehydes in this batch than in the other ones. Therefore, NaCl acts as
a pro-oxidizing and solubilizing agent, increasing the relative levels of volatile compounds from lipid oxidation, among others.
The results obtained in this study indicate that partial NaCl replacement by other chloride salts has an impact on the formation
of volatile compounds in dry-cured “lacón”. Finally, the odor intensity was directly related to the amount of total volatile
compounds.
ACKNOWLEDGEMENTSTOP
The authors are grateful to The Xunta de Galicia (The Regional Government) (Project FEADER 2012/45) for financial support.
Special thanks go to GISVA, S.A. (Arteixo, A Coruña) for the lacón samples supplied for this research.
REFERENCESTOP
| ○ |
Aliño M, Grau R, Baigts D, Barat J. 2009. Influence of sodium replacement on the salting kinetics of pork loin. J. Food Eng. 95, 551–557. http://dx.doi.org/10.1016/j.jfoodeng.2009.06.016.
|
| ○ |
Andrés AI, Cava R, Ventanas S, Muriel E, Ruiz J. 2007. Effect of salt content and processing conditions on volatile compounds
formation throughout the ripening of Iberian ham. Eur. Food Res. Technol. 225, 677–684. http://dx.doi.org/10.1007/s00217-006-0465-z.
|
| ○ |
Armenteros M, Aristoy M, Barat J, Toldrá F. 2009. Biochemical changes in dry-cured loins salted with partial replacements
of NaCl by KCl. Food Chem. 117, 627–633. http://dx.doi.org/10.1016/j.foodchem.2009.04.056.
|
| ○ |
Armenteros M, Aristoy MC, Barat JM, Toldrá F. 2012a. Biochemical and sensory changes in dry-cured ham salted with partial
replacements of NaCl by other chloride salts. Meat Sci. 90, 361–367. http://dx.doi.org/10.1016/j.meatsci.2011.07.023.
|
| ○ |
Armenteros M, Toldrá F, Aristoy MC, Ventanas J, Estévez M. 2012b. Effect of the partial replacement of sodium chloride by
other salts on the formation of volatile compounds during ripening of dry-cured ham. J. Agric. Food Chem. 60, 7607–7615. http://dx.doi.org/10.1021/jf3013772.
|
| ○ |
Association of Official Analytical Chemistry (AOAC) 2005. Official methods of analysis of AOAC International (17th ed.). Maryland, USA.
|
| ○ |
Bermúdez R, Franco D, Carballo J, Lorenzo JM. 2015. Influence of type of muscle on volatile compounds throughout the manufacture
of Celta dry-cured ham. Food Sci. Technol. Int. 21, 581–592. http://dx.doi.org/10.1177/1082013214554935.
|
| ○ |
Blesa E, Aliño M, Barat JM, Grau R, Toldrá F, Pagán MJ. 2008. Microbiology and physico-chemical changes of dry-cured ham during
the post-salting stage as affected by partial replacement of NaCl by other salts. Meat Sci. 78, 135–142. http://dx.doi.org/10.1016/j.meatsci.2007.07.008.
|
| ○ |
Domínguez R, Gómez M, Fonseca S, Lorenzo JM. 2014. Influence of thermal treatment on formation of volatile compounds, cooking
loss and lipid oxidation in foal meat. LWT-Food Sci. Technol. 58, 439–445. http://dx.doi.org/10.1016/j.lwt.2014.04.006.
|
| ○ |
Du M, Ahn DU. 2005. Mechanism of lipid peroxidation in meat and meat products – A review. Food Sci. Biotechnol. 14, 152–163.
|
| ○ |
EFSA 2005.Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related
to the tolerable upper intake level of sodium (Request N EFSA-Q-2003–018). The EFSA J. 209, 1–26.
|
| ○ |
Estévez M. 2011. Protein carbonyls in meat systems: A review. Meat Sci. 89, 259–279. http://dx.doi.org/10.1016/j.meatsci.2011.04.025.
|
| ○ |
Fay LB, Brevard H. 2005. Contribution of mass spectrometry to the study of the Maillard reaction in food. Mass Spectrom Rev. 24, 487–507. http://dx.doi.org/10.1002/mas.20028.
|
| ○ |
Garrido R, Domínguez R, Lorenzo JM, Franco I, Carballo J. 2012. Effect of the length of salting time on the proteolytic changes
in dry-cured lacón during ripening and on the sensory characteristics of the final product. Food Control 25, 789–796. http://dx.doi.org/10.1016/j.foodcont.2011.11.036.
|
| ○ |
Gimeno O, Astiasarán I, Bello J. 1999.Influence of partial replacement of NaCl with KCl and CaCl2 on texture and colour of dry fermented sausages. J. Agric. Food Chem. 47, 873–877. http://dx.doi.org/10.1021/jf980597q.
|
| ○ |
Gómez M, Lorenzo JM. 2013. Effect of fat level on physicochemical, volatile compounds and sensory characteristics of dry-ripened
“chorizo” from Celta pig breed. Meat Sci. 95, 658–666.http://dx.doi.org/10.1016/j.meatsci.2013.06.005.
|
| ○ |
IBM Corp. 2010. IBM SPSS statistics for Windows, version 19.0. New York, NY, USA.
|
| ○ |
Lorenzo JM, Prieto B, Carballo J, Franco I. 2003. Compositional and degradative changes during the manufacture of dry-cured “lacón”. J. Sci. Food Agric. 83, 593–601. http://dx.doi.org/10.1002/jsfa.1375.
|
| ○ |
Lorenzo JM, García-Fontán MC, Franco I, Carballo J. 2008. Biochemical characteristics of dry-cured lacón (a Spanish traditional
meat product) throughout the manufacture, and sensorial properties of the final product.Effect of some additives. Food Control 19, 1148–1158. http://dx.doi.org/10.1016/j.foodcont.2007.12.005.
|
| ○ |
Lorenzo JM. 2014. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured
foal “cecina”. Meat Sci. 96, 256–263. http://dx.doi.org/10.1016/j.meatsci.2013.06.026.
|
| ○ |
Lorenzo JM, Domínguez R. 2014. Cooking losses, lipid oxidation and formation of volatile compounds in foal meat as affected
by cooking procedure. Flavour Frag. J. 29, 240–248. http://dx.doi.org/10.1002/ffj.3201.
|
| ○ |
Lorenzo JM, Fonseca S. 2014. Volatile compounds of Celta dry-cured ‘lacón’ as affected by cross-breeding with Duroc and Landrace
genotypes. J. Sci. Food Agric. 94, 2978–2985. http://dx.doi.org/10.1002/jsfa.6643.
|
| ○ |
Lorenzo JM, Franco D, Carballo J. 2014. Effect of the inclusion of chestnut in the finishing diet on volatile compounds during
the manufacture of dry-cured “Lacón” from Celta pig breed. Meat Sci. 96, 211–223. http://dx.doi.org/10.1016/j.meatsci.2013.07.007.
|
| ○ |
Lorenzo JM, Bermúdez R, Domínguez R, Guiotto A, Franco D, Purriños L. 2015. Physicochemical and microbial changes during the
manufacturing process of dry-cured lacón salted with potassium, calcium and magnesium chloride as a partial replacement for
sodium chloride. Food Control 50, 763–769. http://dx.doi.org/10.1016/j.foodcont.2014.10.019.
|
| ○ |
Lorenzo JM, Carballo J. 2015. Changes in Physico-Chemical properties and Volatile Compounds throughout the Manufacturing Process
of Dry-cured foal Loin. Meat Sci. 99, 44–51. http://dx.doi.org/10.1016/j.meatsci.2014.08.013.
|
| ○ |
Official Journal of the European Communities 2001. Commission Regulation (EC) No 898/2001, of 7 May 2001. L 126, Vol. 44. pp. 18.
|
| ○ |
Purriños L, Bermúdez R, Franco D, Carballo J, Lorenzo JM. 2011a. Development of Volatile Compounds during the Manufacture of Dry-Cured “Lacón,” Spanish Traditional Meat Product. J. Food Sci. 76, 89–97. http://dx.doi.org/10.1111/j.1750-3841.2010.01955.x.
|
| ○ |
Purriños L, Bermúdez R, Temperán S, Franco D, Carballo J, Lorenzo JM. 2011b. Influence of salt content and processing time
on sensory characteristics of cooked “lacón”. Meat Sci. 87, 436–442. http://dx.doi.org/10.1016/j.meatsci.2010.11.022.
|
| ○ |
Purriños L, Franco D, Carballo J, Lorenzo JM. 2012. Influence of the salting time on volatile compounds during the manufacture
of dry-cured pork shoulder “lacón”. Meat Sci. 92, 627–634. http://dx.doi.org/10.1016/j.meatsci.2012.06.010.
|
| ○ |
Purriños L, García-Fontán MC, Carballo J, Lorenzo JM. 2013. Study of the counts, species and characteristics of the yeast
population during the manufacture of dry-cured “lacón”. Effect of salt level. Food Microbiol. 34, 12–18. http://dx.doi.org/10.1016/j.fm.2012.11.003.
|
| ○ |
Ruíz J, Muriel E, Ventanas J. 2002. The flavour of Iberian ham,in Toldrá F. (Ed.) Research advances in the quality of meat and meat products. Trivandrum, India, pp. 289–310.
|
| ○ |
Węsierska E, Szołtysik M, Rak L. 2013. Physico-chemical, biochemical and microbiological properties of traditional Polish
pork fermented products during ripening. Food Bioprocess Tech. 6, 2986–2995.http://dx.doi.org/10.1007/s11947-012-0941-3.
|
| ○ |
WHO/FAO (World Health Organization/Food, Agriculture Organization) 2003. Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series 916. World Health Organization, Geneva, Switzerland, 149.
|
| ○ |
Wu H, Zhang Y, Long M, Tang J, Yu X, Wang J, Zhang J. 2014. Proteolysis and sensory properties of dry-cured bacon as affected
by the partial substitution of sodium chloride with potassium chloride. Meat Sci. 96, 1325–1331. http://dx.doi.org/10.1016/j.meatsci.2013.10.037.
|
| ○ |
Wu H, Zhuang H, Zhang Y, Tang J, Yu X, Long M, Zhang J. 2015. Influence of partial replacement of NaCl with KCl on profiles
of volatile compounds in dry-cured bacon during processing. Food Chem. 172, 391–399. http://dx.doi.org/10.1016/j.foodchem.2014.09.088.
|
| ○ |
Zanardi E, Ghidini S, Conter M, Ianieri A. 2010. Mineral composition of Italian salami and effect of NaCl partial replacement
on compositional, physico-chemical and sensory parameters. Meat Sci. 86, 742–747. http://dx.doi.org/10.1016/j.meatsci.2010.06.015.
|
Figure 1. Evolution of volatile compounds throughtout dry-cured “lacón” processing using different salting formulations. Error bars
indicate the standard error for each treatment. Different letters indicate significant differences among formulations (P<0.05). Plotted values are the means of four replicates.