Effect of dry salting on flavonoid profile and antioxidant capacity of Algerian olive cultivars
O. Soufia,*, C. Romerob, M.J. Motilvac, X. Borrás Gayac and H. Louailechea
aLaboratoire de Biochimie Appliquée, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, 06000 Bejaia, Algérie
bFood Biotechnology Department, Instituto de la Grasa (IG-CSIC), Ctra. de Utrera km1, Campus Universitario Pablo de Olavide,
Edificio 46, 41013 Sevilla, Spain
cDepartment of Food Technology CeRTA-TPV, Escuela Técnica Superior de Ingeniería Agraria, Universidad de Lleida, Spain
*Corresponding author: souficqa@yahoo.fr
| |
SUMMARY
This study investigated the changes in the flavonoid profile and antioxidant capacity of five olive cultivars after dry salting.
The antioxidant activity was determined using ferric reducing ability power (FRAP), oxygen radical absorbance capacity (ORAC),
and β-carotene bleaching assays. The results showed that the effects of dry salting on the analyzed parameters were significant
(P<0.05). It caused a decrease in total flavonoids with a loss rate of 55%. The HPLC analysis of extracts revealed the presence
of four flavonoids: rutin, luteolin-7-glucoside, cyanidin-3-glucoside and cyanidin-3-rutinoside. Among the studied cultivars,
Azeradj was characterized by high levels of flavonoids. Concerning the antioxidant activity, diverging results were obtained
using different antioxidant assays. Overall, the dry salting induced a reduction in the antioxidant activity with variable
values depending on the cultivar. Among the used methods, high correlations were found between flavonoid contents and the
FRAP assay.
|
| |
RESUMEN
Efecto de la salazón en seco sobre el perfil de flavonoides y la capacidad antioxidante de cultivares Argelinos. En este estudio se investigó los cambios en el perfil de flavonoides y la capacidad antioxidante de cinco cultivares de
olivo después de una salazón en seco. La actividad antioxidante se determinó mediante los métodos FRAP (Ferric ion Reducing
Antioxidant Power), ORAC (capacidad de absorción de radicales de oxígeno) y ensayos de blanqueo de β-caroteno. Los resultados
mostraron que los efectos de la salazón en seco en los parámetros analizados fueron significativos (P<0,05). Esto causó una disminución en los flavonoides totales con una tasa de pérdida del 55%. El análisis por HPLC de los
extractos reveló la presencia de cuatro flavonoides: rutina, luteolina-7-glucósido, cianidina-3-glucósido y cianidina-3-rutinósido.
Entre los cultivares estudiados, Azeradj se caracteriza por altos niveles de flavonoides. En cuanto a la actividad antioxidante,
se obtuvieron resultados divergentes utilizando diferentes ensayos antioxidantes. En general, la salazón en seco indujo una
reducción en la actividad.
|
1. INTRODUCTIONTOP
The olive tree (Olea europaea) is widely cultivated in many regions of the world where climatic conditions are as favorable as those prevailing in the
Mediterranean countries. During the last decade, the evolution of the Algerian market concerning table olives was characterized
by a production that has evolved in a fluctuating trend of one olive crop to another. Algeria’s olive crop area was around
188,923 ha by 2011. The total table olive production was estimated to be 192,785 tons in 2011 (ITAFV, 2011). The olives cultivated in Algeria belong to a wide range of cultivars including Azeradj, Bouchouk, Aberkane and Atefah.
In recent years, particular attention has been focused on the specific olive polyphenols which constitute a complex mixture
of flavonoid and non-flavonoid compounds. Romero et al. (2002a) identified the olive flavonoids as quercetin-3-O-rutinoside (rutin), luteolin-7-glucoside, quercetin-3-rhamnoside, cyanidin-3-glucoside and cyanidin-3-rutinoside. These substances
contribute to the total antioxidant potential of the diet and thus may lower the risk of cancer and some chronic diseases.
The flavonoids inhibit lipid peroxidation and exhibit various physiological activities, including anti-inflammatory, anti-allergic,
anti-carcinogenic, anti-hypertensive and anti-arthritic activities (Erlund, 2004). Recently, Dhanya et al. (2014) demonstrated that quercetin and the aglycone of rutin are considered as dietary supplements with potential for the prevention
and treatment of type 2 diabetes and to suppress oxidative stress-mediated damage in diabetic pathophysiology.
In a previous investigation, we have demonstrated that elaboration with dry salt significantly affects the ortho-diphenol profile of six black olive cultivars and the antioxidant capacity of the final product (Soufi et al., 2014). In the current study, we aim to analyze another class of phenolics which are the flavonoids of five Algerian olive cultivars,
in order to obtain a more accurate estimation of olive polyphenols. Total flavonoid contents were also determined to establish
a relationship between these classes of compounds and the antioxidant activity of both fresh and salted olives. As far as
we know, this report is the first to focus on the flavonoid composition of Algerian olive cultivars, particularly after dry
salting.
Since olive polyphenols have multiple characteristics, no single assay available provides all of the information desired.
For this reason, the evaluation of overall antioxidant capacity may require multiple assays. Hence, this work aims also to
use three methods (FRAP, ORAC and β-carotene bleaching) based on different mechanisms to estimate the antioxidant capacity
of both fresh and salted olives.
2. MATERIALS AND METHODSTOP
2.1. Olive samplesTOP
Five black olive cultivars (Azeradj, Bouchouk, Abelout, Aberkane and Atefah) harvested at the fully ripe stage were hand-picked
from different parts of olive trees in the Bejaia location (north of Algeria), in December, 2010.
2.2. Processing of olive samplesTOP
The collected olives (at least 2 Kg) were treated with alternating layers of dry salt (0.8 Kg), in baskets, and kept at room
temperature for 30 to 50 days depending on the cultivar (Panagou, 2006). The salting caused dehydration and the olives appeared shriveled. The fresh and salted olive pulps were freeze-dried (Christ,
Alpha 1–4 LDplus, Osterode am Harz, Germany), then ground in an electric blender (IKA model A 11 B, Staufen, Germany) and
stored at −18 °C until analysis.
2.3. Extract preparationTOP
Freeze dried olive pulp (100 mg) was homogenized with 10 mL of 50% acetone. After stirring for 30 min, the mixture was centrifuged
(nüve NF 200, Ankara, Turkey) at 2800x g for 20 min. The supernatant was collected and filtered, and the residue was re-extracted. The filtered extracts were combined,
washed with hexane (5×10 mL), and then kept in the refrigerator until analysis (McDonald et al., 2001).
2.4. Flavonoid analysisTOP
2.4.1. Total flavonoidsTOP
Total flavonoid contents were determined according to the procedure of Kim et al. (2003). An aliquot of sample (200μL) was mixed with distillated water (800 μL). A volume of 60 μL of 5% NaNO2 was added to the flask. After 5 min, 60 μL of 10% AlCl3 were added. At 6 min, 40 μL of NaOH (1 M) were added to the mixture. Immediately, the contents of the reaction flask were
diluted with the addition of 480 μL of water and thoroughly mixed. Absorbance of the mixture was measured at 510 nm. Catechin
was used as standard and the amount of flavonoids was calculated as milligrams of catechin equivalents (CE) per 100 g of dry
weight.
2.4.2. Individual flavonoidsTOP
2.4.2.1. Non-anthocyanin compoundsTOP
The preparation of extracts was based on the methodology proposed by Sánchez et al. (2013). Freeze dried olive pulp (1 g) was homogenized with 6 mL of dimethylsulfoxide (DMSO). After stirring for 2 min, the mixture
was centrifuged at 28000×g for 6 min at 22 °C; the supernatant was collected and filtered through a 0.22 μm nylon filter. An aliquot of filtrate (250
μL) was diluted with 500 μL of DMSO and 250 μL of 0.2 mM syringic acid (internal standard). A volume of this mixture (20 μL)
was injected for HPLC analysis; a flow rate of 1 mL·min−1 and a temperature of 35 °C were used.
The HPLC system consisted of a Waters 717 plus autosampler, a Waters 600 E pump, a Waters column heater module, and a Waters
996 photodiode array detector operated with Empower software (Waters Inc). A 25 cm×4.6 mm i.d., 5 μm, Spherisorb ODS-2 (Waters
Inc.) column was used. The separation was achieved by gradient elution using an initial composition of 90% water (pH 2.5 adjusted
with 0.15% phosphoric acid) and 10% methanol. The concentration of the latter solvent was increased to 30% in 10 min and maintained
for 20 min. Subsequently, the methanol percentage was raised to 40% in 10 min, which was maintained for 5 min. Finally, the
methanol concentration for the last three steps was increased to 60, 70, and 100% in 5 min periods. Initial conditions were
reached in 15 min. Chromatograms were recorded at 280 nm (Romero et al., 2002b). The concentration of each compound was calculated using a standard curve. Luteolin 7-O-glucoside and rutin were purchased from Extrasynthese SA (Lyon Nord, Genay, France).
2.4.2.2. Anthocyanin compoundsTOP
Extraction was based on the methodology proposed by Romero et al. (2002a). Anthocyanins were extracted from 4 g of freeze-dried olive pulp using 6×30 mL of methanol:hydrochloric acid (99:1, V/V)
at 0 °C. The mixture was stirred for 1 min then centrifuged at 9000 g during 6 min (10 °C). A volume of 20 mL of a solution
(water:hydrochloric acid 99/1, V/V) was added to the methanolic extract which was then concentrated under vacuum at 30 °C
until water residue then transferred to a 25 mL flask with acidified water. A washing step with 4×50 mL of hexane was carried
out to remove the fat from the extract. Finally, the extract was filtered through a 0.22 μm filter and injected into HPLC.
The HPLC system consisted of a Waters 2695 Alliance with a pump, column heater, and autosampler modules included-Detection
was carried out with a Waters 996 photodiode array detector. The system was controlled with Millennium software (Waters Inc.,
Milford, MA) A 25 cm×4.6 mm i.d., 5-μm Extrasil ODS-2 (Technokroma, Barcelona, Spain) column was used and the elution conditions
were as follows: flow rate=1 mL·min–1; column temperature 40 °C, sample temperature 10 °C, solvent A, water with 1% perchloric acid, solvent B, methanol. The mobile
phase consisted initially of 80% of A; using a linear gradient, the concentration of methanol was increased to 50% over 35
min, to 98% at 40 min, held for 2 min at 98% of B to wash the column, and then returned to the initial conditions (20% of
B) for 10 min. Chromatograms were recorded at 520 nm. The evaluation of each anthocyanin compound was performed using a four-point
regression curve obtained using the available standards; cyanindin-3-O-glucoside and cyanidin-3-O-rutinoside were purchased from Extrasynthese S.A. (Lyon Nord, Genay, France).
2.5. Antioxidant activityTOP
2.5.1. FRAP assayTOP
The ferric reducing antioxidant power (FRAP) was applied as described by Benzie and Strain (1996). A volume of acetate buffer (160 μL) was added to 20 μL of sample. The mixture was placed in a well plate and put in the
spectrophotometer (Thermo Scientific, Madrid, Spain) at 37 °C, then mixed during 2min. After that, 40 μL of FeCl3 (10 mM) and 40 μL of 2,4,6-tripyridyl-s-triazine TPTZ (33%) were added, and the absorbance was recorded at 593 nm after 10
min. The results were calculated and related to a Fe+2 standard solution tested in parallel and expressed in micromol of FeSO4 per gram of dry weight (μmol of Fe+2·g–1 dw).
2.5.2. Hydrophilic ORACTOP
The hydrophilic oxygen radical absorbance capacity (ORAC) assay is limited to the measurement of the hydrophilic chain breaking
against peroxyl radicals. A further dilution of the olive extract was made with phosphate buffer. A portion of 25 μL of the
diluted sample was added to a well in a 48-well microplate. A volume of 180 μL of fluorescein solution (0.45 mg·mL–1) and 75 μL of the 2,2′-azobis (2-amidino-propane) dihydrochloride AAPH (60 mg·mL–1) were added to the assay mixture. ORAC values were calculated and expressed as micromol of trolox equivalents per gram of
dry weight (μmol of TE·g–1 dw) (Prior et al., 2003).
2.5.3. β-Carotene bleaching assayTOP
Antioxidant activity was estimated according to the procedure described by Velioglu et al. (1998). A β-carotene solution was prepared in chloroform (0.12 mg·mL–1). Next, 3 mL were taken and added to a flask containing 40 mg of linoleic acid in 400 mg of tween 20. The chloroform was
removed in a vacuum evaporator, and then 100 mL of hydrogen peroxide (30%) were added. After thorough mixing, 3 mL of the
emulsion were added to 0.5 mL of extract (without washing with hexane).
The oxidation of β-carotene emulsion was monitored at 470 nm after incubation at 50 °C (120 min). The antioxidant activity
was expressed as percent inhibition relative to the control using the following equation:
AA = (Rcontrol–Rsample)×100/Rcontrol
Where Rcontrol and Rsample were the bleaching rates of β-carotene in the reactant mixture without antioxidants and with olive extracts, respectively.
2.6. Statistical analysisTOP
Results were expressed as means±standard deviation (SD). The statistical analysis of the data was carried out with STATISTICA
5.5 Fr. Analysis of variance (ANOVA) was performed to estimate the statistically significant differences among the olive samples
for each parameter. P values <0.05 were regarded as significant.
3. RESULTS AND DISCUSSIONTOP
3.1. Flavonoid analysisTOP
3.1.1. Total flavonoidsTOP
The results showed significant differences (P<0.05) in the total flavonoid contents among the studied cultivars (Table 1). The total flavonoid concentration of cultivars ranged between a mean value of 872 (Aberkane and Abelout) and 1537 mg CE·100g–1dw (Azeradj) in fresh olives. These contents are higher than those obtained by Brahmi et al. (2013) who used methanol as the extraction solvent. However, the flavonoid amounts are comprised only between 394 (Abelout) and
1272 mg CE·100 g–1dw (Azeradj) in salted olives. Consequently, the dry salting caused a decrease in flavonoid contents with a loss rate ranging
from 22% (Azeradj) to a mean value of 55% (Abelout and Bouchouk). This decrease can be explained by the diffusion of these
compounds under the action of salt and/or their oxidation during salting. Furthermore, the variability of the decrease noted
among the studied cultivars can be related to the characteristics of each cultivar such as diameter of fruit, since the decrease
is related to the diffusion of such compounds (Bianchi, 2003). In addition, the difference of the polarity of each flavonoid compound can also influence their diffusion (Tomás-Barberán and Gil, 2008).
Table 1. Total flavonoid contents of the studied olives
| Cultivar |
Code |
Total flavonoids1 |
| Fresh olives |
salted olives |
| Azeradj |
AZ |
1537±68aA |
1272±82aB |
| Abelout |
BT |
844±20dA |
394±17dB |
| Aberkane |
BK |
902±8dA |
608±38bB |
| Atefah |
T |
1038±44cA |
593±27bcB |
| Bouchouk |
B |
1149±28Ba |
493±23cdB |
| A and B: within the same row (effect of processing), different letters indicate statistically significant differences (p<0.05).
|
| a-d: Within the same column, different letters indicate statistically significant differences (p<0.05) among cultivars.
|
| 1Results in mg CE·100 g–1dw are expressed as the average± Standard deviation of three replicates.
|
3.1.2. Individual flavonoidsTOP
The analysis of the studied cultivars showed considerable quantitative differences (P<0.05) in individual flavonoids (Table 2). Four flavonoids were identified: rutin, luteolin-7-O-glucoside, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside. The presence of rutin and luteolin-7-glucoside in olive fruits was always reported (Brenes et al., 1995; Morello et al., 2005; Savarese et al., 2007) except that the fresh olives in the present study did not contain the luteolin-7-glucoside until they were treated. Also, Romero et al. (2002a) identified cyanidin-3-O-glucoside and the cyanidin-3-O-rutinoside as the main pigments in seven natural black olive cultivars.
Table 2. Individual flavonoids1 of fresh and salted olives evaluated by HPLC-DAD
| Flavonoid compound Fresh olives |
Azeradj |
Abelout |
Aberkane |
Atefah |
Bouchouk |
| Luteolin-7-Glucoside |
nd |
nd |
nd |
nd |
nd |
| Quercetin-3-rutinoside (rutin) |
1857.16±79.72aA |
227.63±22.41cA |
410.73±0.41bA |
461.94±61.96bA |
454.27±61.52bA |
| Cyanidin-3-glucoside |
2587.98±48.58a |
527.65±07.00b |
242.79±02.65c |
03.50±00.58d |
28.55±01.53d |
| Cyanidin-3-rutinoside |
4358.09±24.38aA |
966.60±02.70bA |
751.98±37.89cA |
27.26±04.05eA |
78.85±04.41dA |
| Salted olives |
| Luteolin-7-glucoside |
370.54±48.92a |
29.81±5.94d |
122.96±08.94c |
300.11±25.35b |
116.15±8.33c |
| Quercetin-3-rutinoside (rutin) |
473.89±59.84aB |
24.76±04.66cB |
94.87±10.44bB |
91.51±00.73bB |
84.26±04.12bB |
| Cyanidin-3-glucoside |
nd |
nd |
nd |
nd |
nd |
| Cyanidin-3-rutinoside |
16.70±04.81aB |
11.50±02.06bB |
07.16±01.27cB |
nd |
nd |
| 1Results are expressed in mg·Kg–1 of dried weight±standard deviation; for each row, different letters (a–d) indicate statistically significant differences (ANOVA test, P<0.05) among cultivars; n.d.: not detected; A and B: within the same column for each compound (effect of processing).
|
The amount of rutin in fresh olives varied between 227 (Abelout) and 1857 mg·Kg–1dw (Azeradj). These contents are similar to those found by Sousa et al. (2014) and Morello et al. (2005) except for the Azeradj cultivar which contain a high concentration. This finding is similar to that reported by Garrido-Fernández et al. (1997) who consider that the flavonoid composition is useful for the biochemical characterization of olive cultivars.
The content of cyanidin-3-glucoside in fresh olives varied from a mean value of 16 (Bouchouk and Atefah) to 2588 mg·Kg–1dw (Azeradj), whereas, the concentration of cyanidin-3-rutinoside is comprised between 27 (Atefah) and 4358 mg·Kg–1dw (Azeradj). These amounts are relatively higher than those obtained by Romero et al. (2002a). The olive cultivars of the present study had the characteristic that the cyanidin-3-glucoside amount is higher than that
of cyanidin-3-rutinoside. This could represent a useful tool for a phytochemical characterization of the cultivars.
After processing, we noted a decrease in individual flavonoid contents except for luteolin-7-glucoside. The content of luteolin-7-glucoside
ranged from 30 (Abelout) to 370 mg·Kg–1dw (Azeradj). These amounts are higher than the luteolin content reported by Dimitrios (2006) for Greek-style naturally black olives (25–75 mg·Kg–1dw), although the rutin content ranged between 24 (Abelout) and 474 mg·Kg–1dw (Azeradj). Piscopo et al. (2014) reported a decrease in the quercetin amount after the drying of green olives. Also, Brenes et al. (1995) noted that the rutin content of the olive flesh decreased with the alkaline treatment, and practically disappeared after
the washing step. In the adopted method, the content of this compound decreased but it did not disappear. This reduction can
be attributed to the glycosidic bond breaking during salting.
The obtained results indicate that the effect of dry salting is dependent on the individual flavonoids; it can induce a decrease
(rutin) or an increase (luteolin-7-glucoside). This is in agreement with the data reported by Rice-Evans and Packer (2003), since salt can generate sodium adducts from flavonol-3-glucoside (rutin), and consequently, the content of the latter decreases.
By contrast, these adducts are not obtained from flavone glucoside (luteolin-7-glucoside).
The dry salting significantly affects (P<0.05) the content of olive pigments: the cyanidin-3-glucoside disappeared, but the cyanidin-3-rutinoside is detected only
in three cultivars with concentrations of 7 (Aberkane), 11 (Abelout) and 16 mg/Kg dw (Azeradj). This can be explained by the
fact that anthocyanins are water-soluble compounds which diffused from the olive to the surrounding medium during dry salting.
These substances can also be either transformed or degraded during processing. According to Garrido-Fernández et al. (1997), the anthocyanin contents may be strongly influenced by the processing and the cultivar; the total content can decrease
to below 50% of its initial value.
The cultivar had a significant effect on the observed changes in the flavonoid composition of studied olives. Among the investigated
cultivars, for both fresh and salted olives, Azeradj showed the highest flavonoid levels and even higher than that of other
studies on olive cultivars (Morello et al., 2005; Damak et al., 2008).
3.2. Antioxidant activityTOP
3.2.1. FRAP assayTOP
The antioxidant capacity of olive extracts is determined by the ability of the antioxidants to reduce ferric to ferrous iron.
The statistical analysis showed significant differences (P<0.05) in the FRAP values of the studied olive cultivars. They varied from 126 (Bouchouk) to 353 μmol of Fe+2·g–1dw (Azeradj) for fresh olives, while these activities ranged between 127 (Bouchouk) and 209 μmol of Fe+2·g–1dw (Azeradj) for salted ones (Figure 1). These data reveal that both fresh and salted olive extracts showed a marked capacity for iron reduction with values higher
than those obtained by Ziogas et al. (2010). The ferric reducing antioxidant power of both fresh and salted olive cultivars decreased in the following order: Azeradj>Abelout=Aberkane>Atefah>Bouchouk.
The processing had a significant effect (P<0.05) on this activity. Overall, we noted a decrease in ferric reducing capacity except for Bouchouk cultivar, which showed
a stable activity. The decrease varied between a mean value of 16% (Abelout and Aberkane) and 41% (Azeradj). This result could
be explained by the low contents of flavonoids and/or other reducing agents in salted olives, since, the antioxidant capacity
of flavonoids has been attributed to their electron-donating ability (Morales-Soto et al., 2014). Also, Prior et al. (2005), consider that the reducing capacity is related to the degree of hydroxylation which is a characteristic of flavonoid compounds.
The present study demonstrated that other factors such as the structure of antioxidant could also influence this activity,
since Azeradj cultivar which had the lowest loss rate of flavonoids, lost almost half of its activity, while inverse effect
was noted for the Abelout cultivar.
|
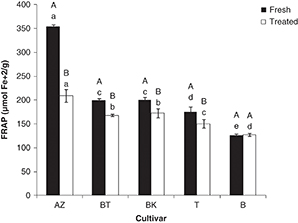 Figure 1. Ferric Reducing Antioxidant Power of olive cultivars. Effect of salting (A- B): different letters indicate statistically significant differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1). Figure 1. Ferric Reducing Antioxidant Power of olive cultivars. Effect of salting (A- B): different letters indicate statistically significant differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1).
|
|
3.2.2. Hydrophilic ORAC (ORACH)TOP
Figure 2 shows significant differences in antioxidant activity among the studied cultivars (P<0.05): ORACH values ranged between 201 (Bouchouk) and 551 μmol TE·g–1dw (Azeradj) in fresh olives; these values varied from 199 (Atefah) to 418 μmol TE·g–1dw (Aberkane) in salted ones.
|
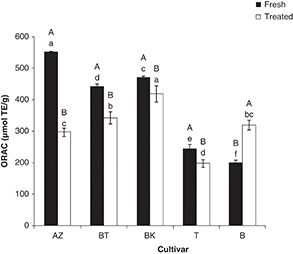 Figure 2. Oxygen absorbing Power Capacity of olive cultivars. Effect of salting (A- B): different letters indicate statistically significant differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1). Figure 2. Oxygen absorbing Power Capacity of olive cultivars. Effect of salting (A- B): different letters indicate statistically significant differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1).
|
|
The obtained results indicate that dry salting had a significant effect on the antioxidant capacity (P<0.05); it leads to a decrease for the studied cultivars except for Bouchouk which showed an increase of 37%. In fact, the loss rate varied from 13 (Aberkane) to 47% (Azeradj). The scavenging effects of fresh olive cultivars against the oxygen radical decreased in the following order: Azeradj > Aberkane > Abelout > Atefah > Bouchouk. After processing, this order changed to the following: Aberkane > Abelout ≥ Bouchouk > Azeradj > Atefah. This variation could be related to the specific changes in phenolic composition of each cultivar after processing, since the ORAC assay cannot be based only on the flavonoids which are effective electron-donors, but also includes the hydrogen donor compounds. On the other hand, the increase noted for Bouchouk cultivar can be explained by the possible synergistic effect between the antioxidant compounds detected after processing.
On the other hand, the results reveal that the cultivar containing high levels of rutin did not show necessarily low ORACH values. This osbervation is different than that of Ou et al. (2002). This can be related to the specific antioxidant composition of the olive cultivar and the possible synergistic effect of
other antioxidants with rutin in the total ORAC assay.
3.2.3. β-Carotene bleaching assayTOP
The antioxidant activity of olive extracts measured by the bleaching of β-carotene is shown in Figure 3. The statistical analysis (P<0.05) revealed that the fresh olive extracts showed an inhibition rate which ranged between 32 (Aberkane) and 54% (Bouchouk).
|
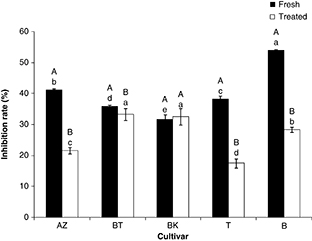 Figure 3. β-Carotene bleaching activity of olive cultivars. Effect of salting (A-B): different letters indicate statistically significant
differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1). Figure 3. β-Carotene bleaching activity of olive cultivars. Effect of salting (A-B): different letters indicate statistically significant
differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1).
|
|
Concerning the treated olives, the inhibition activity varied from 17 (Atefah) to a mean value of 33% (Aberkane and Abelout).
Dry salting significantly affected the β-carotene bleaching activity of the studied olives except for the Aberkane cultivar;
it induced a loss with a rate comprised between 9% (Abelout) and 52% (Atefah). We note that Azeradj cultivar, which showed
high values of ORAC and FRAP, had a relatively low inhibition effect of β-carotene oxidation. This can be related to its low
content in lipophilic antioxidants, which may play a major role in this activity since the extracts in the β-carotene bleaching
assay are not defatted and contain both lipophilic and hydrophilic antioxidants. This observation is similar to that of Alu’datt et al. (2013) who demonstrated that full-fat olive extracts had higher antioxidant activity than de-fatted extracts.
Because multiple reaction characteristics and mechanisms are likely involved, each method only provides an estimate of antioxidant
capacity which is subjective to its conditions and reagents.
3.3. Relationship between flavonoid contents and antioxidant activityTOP
The statistical study showed that the correlation coefficient did not depend only on the applied method, but, also on the
olive processing, which significantly affected its composition (Table 3). A good correlation was obtained between total flavonoid contents and FRAP, thus reflecting the role of such compounds as
reducers of iron. This observation is in accordance with that of Du et al. (2009).
Table 3. Correlation coefficients (R)*for the relationship between the antioxidant compounds and the antioxidant potential measured by FRAP (Ferric Reducing Ability of Plasma), ORAC (Oxygen Radical Absorbance Capacity) and β-carotene bleaching activity (CBA)
| Correlation between |
Fresh olives |
Salted olives |
| (R)* |
Equation |
(R)* |
Equation |
| Flavonoids- FRAP |
0.70 |
y=0.216x-25.8 |
0.78 |
y=0.069x+118.8 |
| Flavonoids- ORAC |
0.24 |
y=0.134x+235.6 |
0.14 |
y=−0.034x+338.7 |
| Flavonoids- CBA |
0.43 |
y=0.013x+25.82 |
0.50 |
y=−0.01x+33.44 |
| Flavonols- FRAP |
0.94 |
y=2.026x-91.0 |
0.86 |
y=0.532x+78.0 |
| Flavonols- ORAC |
0.73 |
y=2.809x-35.8 |
0.23 |
y=0.377x+253.6 |
| Flavonols- CBA |
0.11 |
y=−0.025x+43.91 |
0.03 |
y=−0.005x+27.54 |
| FRAP-ORAC |
0,82 |
y=1.463x+74.0 |
0.17 |
|
| FRAP- CBA |
0.27 |
y=−2.794x+322.7 |
0.10 |
y=−0.462x+178 |
| ORAC- CBA |
0.60 |
y=−10.76x+814.5 |
0.84 |
y=9.647x+58.74 |
| Lut-7-Glu-FRAP |
– |
– |
0.47 |
y=0.143x-0.2 |
| Lut-7-Glu-ORAC |
– |
– |
0.60 |
y=−0.345x+380.6 |
| Lut-7-Glu- CBA |
– |
– |
0.87 |
y=−0.043+34.79 |
| Rutin-FRAP |
0.89 |
y=0115x+131.9 |
0.75 |
y=0.128x+145.8 |
| Rutin-ORAC |
0.54 |
y=0.124x+297.5 |
0.14 |
y=−0.064x+325.7 |
| Rutin- CBA |
0.12 |
y=0.001x+39.12 |
0.46 |
y=−0.017x+29.40 |
| Cy-3-Glu-FRAP |
0.96 |
y=0.075x+159.1 |
0.75 |
y=0.128x+145.8 |
| Cy-3-Glu-ORAC |
0.74 |
Y=0.102x+312.4 |
0.14 |
y=−0.064x+325.7 |
| Cy-3-Glu- CBA |
0.03 |
y=0x+40.37 |
0.46 |
y=−0.017x+29.40 |
| Cy-3-rutin-FRAP |
0.97 |
y=0.046x+153.1 |
0.61 |
y=2.821x+150.1 |
| Cy-3-rutin-ORAC |
0.78 |
y=0.065x+300.7 |
0.83 |
y=−10.12x+472.7 |
| Cy-3-rutin- CBA |
0.08 |
y=0x+40.67 |
0.75 |
y=−0.984x+40.74 |
| *Significant at P≤0.05.
|
| Lut-7-gluc: luteoline-7-glucoside, Cy-3-gluc: Cyanidin-3-glucoside, Cy-3-rut: Cyanidin-3-rutinoside. |
The present study also demonstrated that the correlation between the total flavonoid contents and ORAC of fresh olives is
characterized by higher coefficients than those of salted ones. This can signify that ORAC method is dependent on the flavonoid
concentration since a considerable loss in these compounds occured after processing. On the other hand, a low correlation
was noted between the analyzed compounds (individual flavonoids and total flavonols) and the β-carotene bleaching activity.
These results are in agreement with those obtained by Rufino et al. (2010) who did not find any correlation between antioxidant compounds and β-carotene bleaching activity. This can reflect the contribution
of other compounds rather than flavonoids to this antioxidant capacity and/or variations in the efficiency of individual olive
phenolics or in combination.
The comparison of the antioxidant assays used revealed a good correlation between FRAP and ORAC in fresh olives (r=0.82),
but only a slight one was noted between the same assays in salted olives (r=0.17). This can be explained by the different
antioxidant compositions, since salting had an impact on these compounds and not all antioxidants can act as hydrogen donors
or as iron reducers. In addition, the possibility of a synergistic effect among the antioxidants of fresh olives may explain
the high antioxidant activity coefficient of the olive extracts.
Among the antioxidant activity methods used, the FRAP assay was highly correlated with each flavonoid identified in fresh
or salted olives, although β-carotene bleaching activity appears to be more correlated with luteoline-7-glucoside contents.
4. CONCLUSIONTOP
In the present study, we evaluated the effect of dry salting on the flavonoid profile and antioxidant activity of five Algerian
olive cultivars. We demonstrated that the changes in these compounds and antioxidant capacity occurring after processing depend
on the cultivar. The HPLC analysis of the extracts revealed the presence of four flavonoids: rutin, luteolin-7-glucoside,
cyanidin-3-glucoside and cyanidin-3-rutinoside. Among the antioxidant activity assays used, a good correlation was noted between
FRAP and total flavonoid contents, indicating that these compounds could be among the main constituents responsible for the
reducing ability of olives. An inclusive evaluation of all possible antioxidant activities would require a combination of
several methods since varying results are obtained. This study supplied new information on the antioxidant capacity of olives
for consumers and nutritionists, especially with this kind of process. In this work, we focused on hydrophilic extracts, although
lipophilic components would be necessary to complete the data presented here.
ACKNOWLEDGEMENTSTOP
Thanks are due to Ifri-Olive, Mr Ait Kheddache S. and all those who have harvested the olive samples used in this study, and
to the Algerian Ministry of Higher Education and scientific Research who funded this work. This work was also supported by
project AGL 2009–07512 from the Spanish Government and The European Union (European Regional Development Funds).
REFERENCESTOP
| ○ |
Alu’datt MH, Rababah T, Ereifej K, Alli I. 2013. Distribution, antioxidant and characterisation of phenolic compounds in soybeans,
flaxseed and olives. Food Chem. 139, 93–99. http://dx.doi.org/10.1016/j.foodchem.2012.12.061.
|
| ○ |
Benzie I, Strain J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP Assay.
Anal Biochem. 239, 70–76. http://dx.doi.org/10.1006/abio.1996.0292.
|
| ○ |
Bianchi G. 2003. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 105, 229–242. http://dx.doi.org/10.1002/ejlt.200390046.
|
| ○ |
Brahmi F, Mechri B, Dhibi M, Hammami M. 2013. Variations in phenolic compounds and antiradical scavenging activity of Oleaeuropaealeaves and fruits extracts collected in two different seasons. Ind. Crops Prod. 49, 256–264. http://dx.doi.org/10.1016/j.indcrop.2013.04.042.
|
| ○ |
Brenes M, Rejano L, Garcia P, Sanchez AH, Garrido A. 1995. Biochemical changes in phenolic compounds during spanish-style
green olive processing. J. Agric. Food Chem. 43, 2702–2706. http://dx.doi.org/10.1021/jf00058a028.
|
| ○ |
Damak N, Bouaziz M, Ayadi M, Sayadi S, Damak M. 2008. Effect of the maturation process on the phenolic fractions, fatty acids,
and antioxidant activity of the chétoui olive fruit cultivar. J. Agric. Food Chem. 56, 1560–1566. http://dx.doi.org/10.1021/jf072273k.
|
| ○ |
Dhanya R, Arun KB,Syama HP, Nisha P, Sundaresan A, Santhosh Kumar TR, Jayamurthy P. 2014. Rutin and quercetin enhance glucose
uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 158, 546–554. http://dx.doi.org/10.1016/j.foodchem.2014.02.151.
|
| ○ |
Dimitrios B. 2006. Sources of natural phenolics antioxidants. Trends Food Sci. Tech. 17, 505–512. http://dx.doi.org/10.1016/j.tifs.2006.04.004.
|
| ○ |
Du G, Li M, Ma F, Liang D. 2009. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits.
Food Chem., 113, 557–562. http://dx.doi.org/10.1016/j.foodchem.2008.08.025.
|
| ○ |
Erlund I. 2004. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability,
and epidemiology. Nutr. Res. 24, 851–874. http://dx.doi.org/10.1016/j.nutres.2004.07.005.
|
| ○ |
Garrido-Fernández A, Fernández-Díez MJ, Adams MR. 1997. Table olives: Production and processing. In Olives and table olives
(pp. 10–21). London, UK: Chapman and Hall.
|
| ○ |
ITAFV. 2011. Institut Technique de l’Arboriculture Fruitière et de la Vigne. Statistiques 2011 des olives de table. Département
Etude Direction Générale (Alger).
|
| ○ |
Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. 2003. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 51, 6509–6515. http://dx.doi.org/10.1021/jf0343074.
|
| ○ |
McDonald S, Prenzler PD, Antolovich M, Robards K. 2001. Phenolic content and antioxidant activity of olive extracts. Food Chem. 73, 73–84. http://dx.doi.org/10.1016/S0308-8146(00)00288-0.
|
| ○ |
Morales-Soto A, García-Salas P, Rodríguez-Pérez C, Jiménez-Sánchez C, Cádiz-Gurrea M, Segura-Carretero A, Fernández-Gutiérrez
A. 2014. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 58, 35–46. http://dx.doi.org/10.1016/j.foodres.2014.01.050.
|
| ○ |
Morello JR, Vuorela S, Romero MP, Motilva MJ, Heinonen M. 2005. Antioxidant activity of olive pulp and olive oil phenolic compounds
of the Arbequina cultivar. J. Agric. Food Chem. 53, 2002–2008. http://dx.doi.org/10.1021/jf048386a.
|
| ○ |
Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. 2002. Analysis of antioxidant activities of common vegetables employingoxygen
radical absorbancecapacity(ORAC) andferricreducing antioxidant power (FRAP) assays: a comparativestudy. J. Agric. Food Chem. 50, 3122–3128. http://dx.doi.org/10.1021/jf0116606.
|
| ○ |
Panagou, E. Z. 2006. Greek dry-salted olives: Monitoring the dry-salting process and subsequent physico-chemical and microbiological
profile during storage under different packing conditions at 4 and 20 °C. Food Sci. Technol. 39, 322–329. http://dx.doi.org/10.1016/j.lwt.2005.02.017.
|
| ○ |
Piscopo A, De Bruno A, Zappia A, Poiana M. 2014. Antioxidant activity of dried green olives (Caroleacv.). Food Sci Technol. 58, 49–54. http://dx.doi.org/10.1016/j.lwt.2014.03.013.
|
| ○ |
Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M., Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R. 2003. Assays for hydrophilic
and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food
samples. J. Agric. Food Chem. 51, 3273–3279. http://dx.doi.org/10.1021/jf0262256.
|
| ○ |
Prior R, WuX, Schaich K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and
dietary supplements. J. Agric. Food Chem. 53, 4290–4302. http://dx.doi.org/10.1021/jf0502698.
|
| ○ |
Rice-Evans CA, Packer L. 2003. Flavonoids in Health and Disease. In Pietta, P. & Gardana, C. Flavonoids in herbs (pp. 43–50).
CRC Press.
|
| ○ |
Romero C, García P, Brenes M, García A, Garrido A. 2002a. Phenolic compounds in natural black Spanish olive varieties. Eur. Food Res. Technol. 215, 489–496. http://dx.doi.org/10.1007/s00217-002-0619-6.
|
| ○ |
Romero C, Brenes M, García P, Garrido A. 2002b. Hydroxytyrosol 4-β-D-glucoside, an important phenolic compound in olive fruits
and derived products. J. Agric. Food Chem. 50, 3835–3839. http://dx.doi.org/10.1021/jf011485t.
|
| ○ |
Rufino MM, Alves RE, Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J. 2010. Bioactive compounds and antioxidant
capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 121, 996–1002. http://dx.doi.org/10.1016/j.foodchem.2010.01.037.
|
| ○ |
Sánchez AH, Romero C, Ramírez E, Brenes M. 2013. Storage of mechanically harvested Manzanilla olives under controlled atmospheres.
Postharvest Biol.Tech. 81, 60–65. http://dx.doi.org/10.1016/j.postharvbio.2013.02.015.
|
| ○ |
Savarese M, De Marco E, Sacchi R. 2007. Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry Food Chem. 105, 761–770. http://dx.doi.org/10.1016/j.foodchem.2007.01.037.
|
| ○ |
Soufi O, Romero C, Louaileche H. 2014. Ortho-diphenol profile and antioxidant activity of Algerian black olive cultivars: Effect of dry salting process. Food Chem. 157, 504–510. http://dx.doi.org/10.1016/j.foodchem.2014.02.075.
|
| ○ |
Sousa A, Malheiro R, Casal S, Bento A, Pereira JA. 2014. Antioxidant activity and phenolic composition of Cv. Cobrançosa olives
affected through the maturation process. J. Funct. Foods. 11, 20–29. http://dx.doi.org/10.1016/j.jff.2014.08.024.
|
| ○ |
Tomás-Barberán F.A, Gil M.I. 2008. Improving the Health-Promoting Properties of Fruit and Vegetable Products. Woodhead Publishing Series in Food Science, Technology and Nutrition, CRC Press LLC. pp. 458–461.
|
| ○ |
Velioglu YS, Mazza G, Gao L, Omah BD. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain
products. J. Agric. Food Chem. 46, 4113–4117. http://dx.doi.org/10.1021/jf9801973.
|
| ○ |
Ziogas V, Tanou G, Molassiotis A, Diamantidis G, Vasilakakis M. 2010. Antioxidant and free radical-scavenging activities of
phenolic extracts of olive fruits. Food Chem. 120, 1097–1103. http://dx.doi.org/10.1016/j.foodchem.2009.11.058.
|
Figure 1. Ferric Reducing Antioxidant Power of olive cultivars. Effect of salting (A- B): different letters indicate statistically significant differences (P<0.05). (a-e) Different letters indicate statistically significant differences (P<0.05) among cultivars. BT, BK, AZ, B, T: are codes corresponding to each cultivar (indicated in Table 1).