Recovery of iron after Fenton-like secondary treatment of olive mill wastewater by nano-filtration and low-pressure reverse osmosis membranes
J.M. Ochando-Pulido*, M.D. Víctor-Ortega and A. Martínez-Férez
Chemical Engineering Department, University of Granada, 18071 Granada, Spain
*Corresponding author: jmochandop@ugr.es
| |
SUMMARY
In this work, the performances of novel nano-filtration (NF) and low-pressure reverse osmosis (RO) polymeric membranes were
examined with the aim of recovering the iron used as catalyst in former secondary treatment based on the Fenton-like advanced
oxidation of olive mill wastewater (OMW). Results highlight that both membranes exhibit a good performance towards the rejection
of iron (99.1% for the NF membrane vs. 100% for the low-pressure RO membrane) in the secondary-treated OMW effluent, thus permitting the recovery of iron in the
concentrate stream in order to recycle it back into the oxidation reactor to reduce catalyst consumption. Finally, the permeate
streams could be re-used for irrigation. Major productivity was observed by the selected NF membrane, about 47.4 L/hm2 upon 9 bar, whereas 30.9 L/hm2 could be yielded with the RO membrane under an operating pressure of 8 bar. Moreover, a sensibly lower fouling index was
measured on the NF membrane (0.0072 in contrast with 0.065), which ensures major steady-state performance on this membrane
and a longer service lifetime. This also results in lower required membrane area and membrane plant over dimension (4 modules
in case of RO operation whereas only 2 modules for NF).
|
| |
RESUMEN
Recuperación de hierro tras tratamiento secundario tipo Fenton de agua residual de la industria oleícola por membranas de
nanofiltración y ósmosis inversa de baja presión. En este trabajo, se examinó el rendimiento de membranas modernas de nanofiltración (NF) y ósmosis inversa (OI) poliméricas
con el objetivo de recuperar el hierro utilizado como catalizador en un tratamiento secundario previo de agua residual oleícola
(OMW) basado en oxidación avanzada tipo Fenton. Los resultados ponen de relieven que ambas membranas exhiben buen rendimiento
en cuanto al rechazo de hierro (99.1% para la membrana de NF vs. 100% para la membrana de OI de bajas presiones) en el efluente oleícola tras tratamiento secundario, permitiendo en consecuencia
la recuperación de hierro en la corriente de concentrado para su recirculación de nuevo al reactor de oxidación para reducir
el consumo de catalizador. Finalmente, las corrientes de permeado podrían ser reutilizadas para riego. Por otro lado, la productividad
asegurada por la membrana de NF seleccionada fue mayor, en torno a 47.4 L/hm2 a 9 bar, mientras que 30.9 L/hm2 pudieron ser producidos por la membrana de OI bajo una presión operativa de 8 bar. Además, un índice de fouling sensiblemente
menor fue medido en la membrana de NF (0.0072 en contraste con 0.065), lo que asegura mayor rendimiento en estado estacionario
para esta membrana, y mayor vida de servicio. Además, ello también resultó en una menor área de membrana y sobredimensionamiento
de la planta requeridas (4 módulos en caso de OI mientras que sólo para NF).
|
1. INTRODUCTIONTOP
One of the key tasks of catalytic processes, from the point of view of cost-efficiency, is the recovery and re-use of the
catalyst. This is especially relevant in the case of catalytic treatments aimed for the reclamation of wastewater streams,
in which the low added-value of the treated effluent (purified water) makes it imperative to save as much expense as possible.
In the case of homogeneous catalytic processes, this is even more difficult to achieve. In the case of heterogeneous catalytic
processes, one of the most common solutions can be the fixation of the catalyst to a solid phase. However, this can make the
catalyst lose some of its effectiveness, given that a less perfect mix may be achieved. Moreover, in case of dark media like
wastewater streams, another problem is added to the former, which is the hindrance of the penetration of light, in the case
of photo-catalyzed processes.
In a previous work by the Authors (Ochando-Pulido et al., 2013a and 2014; Stoller et al., 2015), a novel self lab-made TiO2-based ferromagnetic-core photo-catalyst was developed in the framework of the European project PHOTOMEM (Contract FP7-SME-2010-1
no. 262470). The ferromagnetic properties of this catalyst enhanced its recovery back from the wastewater stream by magnetic
traps, enabling its re-use in successive batches, solving the problem of the recovery of the catalyst and thus enhancing the
cost-effectiveness of the process (Ochando-Pulido et al., 2013a and 2014; Stoller et al., 2015).
In this research paper, an alternative is proposed to our previous work, in this case for two-phase olive mill wastewater
(OMW) previously subjected to a secondary treatment based on a homogeneous Fenton-like reaction (Hodaifa et al., 2013a, b; Martínez Nieto, 2011a, b). The goal was to experimentally evaluate the feasibility of the recovery of the iron used as catalyst (ferric chloride)
by means of membrane technology in the Fenton-like advanced oxidation process conducted to enhance the degradation of the
organic matter in OMW. The goal was to re-use the recovered iron, by separating and concentrating it, in order to pump it
back into the Fenton-like reactor to reduce catalyst consumption.
Much effort has been invested to attain novel membranes capable of offering higher technical and economical performances since
the development and commercialization of the first cellulose acetate asymmetric membranes. The availability of new membrane
materials, designs, module configurations and know-how has succeeded in the promotion of credibility among investors (Akdemir
and Ozer, 2009; Stoller, 2008, 2009 and 2011; Turano et al., 2002).
In the last decades, the effluents generated by olive oil industries (OMW) have significantly increased as a result of the
boost of the olive oil agro-industrial sector, also due to the technological conversion into continuous operation centrifugation-based
processes. Currently, average-sized modern olive oil mills operating with the two-phase centrifugation technology by-produce
daily between 10 and 15 m3 of wastewater derived from the vertical centrifugation process, called olive oil washing wastewater (OOW), together with
1 m3 of olive washing wastewater (OWW) per ton of processed olives (Hodaifa et al., 2008; Hodaifa et al., 2013a, b; Martínez-Nieto et al., 2010, 2011a, b; Ochando-Pulido et al., 2012a, b, 2013a, b). This reaches several million cubic meters of OMW each year.
A wide variety of stand-alone and integrated processes for the treatment of OMW have already been proposed and developed but
have not yet led to completely satisfactory results (Borja et al., 2006), such as lagooning or natural evaporation and thermal concentration (Annesini and Gironi, 1991; Paraskeva and Diamadopoulos, 2006), composting (Cegarra et al., 1996; Papadimitriou et al., 1997), treatments with clay (Al-Malah et al., 2000) or with lime (Aktas et al., 2001), biological processes (Ena et al., 2007; Garrido et al., 2002; Marques, 2001; Hodaifa et al., 2008), physico-chemical procedures including coagulation-flocculation (Sarika et al., 2005), electro-coagulation (Inan et al., 2004; Tezcan et al., 2006) and biosorption (Hodaifa et al., 2013a), advanced oxidation processes comprising ozonation (Beltrán et al., 2000), Fenton’s reaction (Martínez-Nieto et al., 2011a; Hodaifa et al., 2013b) and photocatalysis (Sacco et al., 2012), electrochemical treatments (Papastefanakis et al., 2010) and hybrid processes (Grafias et al., 2010; Lafi et al., 2009).
The disposal of the solid waste stream is not the objective of the present work, which aims only at the management problem
related to the reclamation of liquid effluents. Some solutions already proposed for the management of the pomace waste are
for instance adsorption of heavy metals (Baccar et al., 2009), dyes (Akar et al., 2009) and phenols (Stasinakis et al., 2009) as well as composting (Haddadin et al., 2009) or biogas production (Tekin et al., 2000), among others.
Olive oil industries in their current status, typically small, dispersed mills, cannot afford such high treatment costs. In
addition, conventional physicochemical treatments are not effective for the removal of the significant salinity of OMW, reflected
in high electro-conductivity (EC), which presents hazardous salinity levels according to the guidelines established by the
Food and Agricultural Organization (F.A.O.) for irrigation uses.
Several works have been conducted in the past by means of membrane technology with the aim at reducing the organic load of
OMW (Akdemir et al., 2009; Coskun et al., 2010; Turano et al., 2002; Stoller, 2009), but only a few focus on two-phase (Ochando-Pulido et al., 2012a; Ochando-Pulido et al., 2012b). Furthermore, some authors have tried to extract the added-value compounds contained in this effluent, mainly low-molecular-weight
polyphenols and sugars by concentration with membranes (Garcia-Castello et al., 2010; Paraskeva et al., 2007; Russo, 2007).
In this work, two different membranes, one nano-filtration (NF) and a low-pressure reverse osmosis (RO), are examined with
a double aim: the main one is the recovery of the iron used as catalyst in the former Fenton-like secondary treatment of OMW,
but at the same time a secondary goal was the removal of the high EC and remaining organic matter in this secondary-treated
OMW stream.
For this purpose, the adequate operating pressure for both membranes was studied, with an insight into the impacts on both
the productivity and rejection efficiency towards the target species. The fouling issues occurring on both membranes, which
deeply influence the performance and cost-effectiveness of the membrane process, were also analyzed and taken into account
for the membrane plant dimension. Control of fouling is a key parameter in order to increase the profitability of membrane
processes during operation and avoid excessive overdesign of the membrane plants. High fouling rates on the membranes would
rapidly lead to zero flux conditions in an irreversible way in case of iron (Yiantsios and Karabelas, 2002).
Finally, the suitability for reusing the final effluent (permeate stream) in the olive oil production process and therefore
closing the loop was also checked.
2. MATERIALS AND METHODSTOP
2.1. Feedstock: two-phase olive mill wastewaterTOP
Samples of OWW and OOW effluents were collected from several two-phase centrifugation-based olive oil mills in the Andalusian
provinces of Jaén and Granada (Spain) during winter months and rapidly analyzed in the lab and refrigerated for further research
when necessary.
OWW and OOW were mixed in a 1:1 (v/v) proportion to stabilize the average organic matter concentration of the effluent stream
(OMW) entering the treatment system and thus avoiding sensible fluctuations in the COD parameter. After this, OMW was conducted
to a secondary treatment on a pilot scale based on Fenton-like advanced oxidation process. The secondary treatment is described
in detail in former works by the authors (Martínez-Nieto et al., 2010, 2011a, 2011b; Hodaifa et al., 2013a, 2013b). The OMW effluent after the secondary treatment will be hereafter subjected to the final membrane operation.
2.2. Membranes plantTOP
The membrane plant used for the experiments was a bench-scale one supplied by Prozesstechnik GmbH (Basel, Switzerland), provided
with a plate-and-frame module (Figure 1). Flat-sheet RO and NF membranes were selected for the experiments, supplied by GE Water and Process Technologies, with the
characteristics reported in Table 1.
|
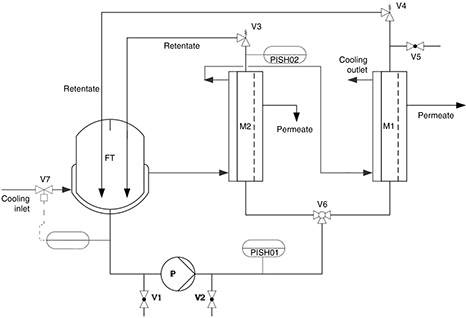 Figure 1. Flow diagram of the bench-scale RO unit. V1, V2: emptying valves (pump inlet and outlet respectively); V3, V4: pressure regulating
valves for module 2 and 1 respectively; V5: venting valve for module M1; V6: three-way valve to select desired working membrane
module; V7: magnetic valve for cooling jacket inlet; M1: flat-sheet membrane module; M2: spiral-wounded module; P: feedstock
pump; FT: feedstock tank; PISH01, PISH02: pressure gauges; TICSH01: temperature gauge. Figure 1. Flow diagram of the bench-scale RO unit. V1, V2: emptying valves (pump inlet and outlet respectively); V3, V4: pressure regulating
valves for module 2 and 1 respectively; V5: venting valve for module M1; V6: three-way valve to select desired working membrane
module; V7: magnetic valve for cooling jacket inlet; M1: flat-sheet membrane module; M2: spiral-wounded module; P: feedstock
pump; FT: feedstock tank; PISH01, PISH02: pressure gauges; TICSH01: temperature gauge.
|
|
Table 1. Nominal characteristics of the selected membranes.
| Parameters |
Parametric value |
| Membrane type
|
NF |
RO |
| Manufacturer
|
GE (USA) |
GE (USA) |
| Model series
|
DK |
AK |
| Permeability (m0), L/hm2bar |
8.2 ± 0.3 |
6.2 ± 0.2 |
| Configuration |
Flat-sheet |
Flat-sheet |
| Chemical composition
|
c TFC a PA/b PS
|
Asymmetric a PA
|
| Average pore size, nm |
0.5 |
- |
| d MWCO, Da |
50 - 300 |
- |
| Maximum pressure, bar |
32 |
8.7 |
| Maximum temperature, °C |
90 |
50 |
| * a PA: polyamide;
|
| b PS: polysulfone;
|
| c TFC: thin-film composite;
|
| MWCO: molecular weight cut-off. |
The membranes plant consists of a non-stirred double walled tank (5 L) and a diaphragm pump (Hydra-Cell model D-03) to drive
the effluent stream to a plate-and-frame membrane module M1 (dimensions 3.9 cm width x 33.5 cm length). The plant is also
provided with another different membrane module (M2), which can be either a spiral-wound or tubular one, and can be selected
with a three-way valve (V6).
The main processing parameters (operating pressure, temperature and feed flow rate) were measured and displayed. The operating
pressure could be adjusted finely with a spring-loaded pressure-regulating valve (SS-R4512MM-SP, Swagelok) on the concentrate
outlet and monitored by a digital pressure gauge (Endress+Hauser, model Ceraphant T PTC31), allowing independent control of
the operating pressure (PTM set point ± 0.01 bar) and the flow rate; the feed flow rate was regulated by means of a feed flow rate valve (Fset point ± 0.1 L/h) to fix the tangential velocity over the membrane (Mott and Untener, 2014); the operating temperature was regulated automatically (Tset point ± 0.1 °C) via a proportional-integral-derivative (PID) electronic temperature controller (Yokogawa model UT100) and a magnetic
valve in the cooling loop, which re-circulates cooling water coming from a chiller (PolyScience model 7306) inside the tank’s
refrigerating jacket. The system is also automatically protected against excess pressure and temperature. All medium wetted
metallic parts are made of stainless steel 316L to avoid corrosion, except the permeate and concentrate stream outlet tubes,
which are made of chemical resistant polyethylene.
2.3. NF and RO performances: experimental procedureTOP
Prior to each NF or RO experiment, the corresponding membrane was stabilized by filtering MilliQ® water at a fixed pressure
and temperature until a constant and stable flux was observed. After this, the hydraulic permeabilities (m0) of each of the selected membranes were determined by measuring the pure water flux over the admissible applied pressures
range of each one, at ambient temperature and turbulent cross-flow velocity.
Subsequently, 2 L of secondary-treated OMW were poured into the feed-water tank to proceed with the experimental OMW membranes
purification. Bench-scale NF and RO experiments were run in a semi-batch mode, conducted in tangential-flow at ambient temperature
(22 ± 0.1 °C) and turbulent regime over the membrane. The operating pressures were fixed at 5, 7 and 9 bars for the experimental
runs with the NF (DK series) membrane, in order to work in a low-pressure energy-saving range, whereas 3, 5 and 8 (maximum
operating pressure 8.7 bar) for the experiments with the RO membrane (AK series), respectively.
All the membrane experiments were run with the highest feed volume recovery possible (Y,%), which is approximately 80 - 90%.
The operating procedure consisted of continuously recycling the concentrate stream back into the feed-water tank where it
steadily collected the permeate stream, which was replaced by the same volume of fresh pretreated OMW. Periodically, samples
of the permeate stream were collected in a cumulative vessel and analyzed in order to evaluate the membrane separation effectiveness
with respect to the iron recovery, as well as COD and conductivity rejection. The membrane productivity was assayed by measuring
the permeate flux during operation time by weighing the mass of collected permeate on a precision electronic mass balance
(AX -120 Cobos, 0.1 mg accuracy).
After each semi-batch run, the membrane was recovered for the following experiment by cleaning it in situ with 0.1-0.15% w/v
NaOH and 0.1-0.15% w/v sodium dodecyl sulfate (SDS) solutions (provided by Panreac S.A.).
The membrane performances were measured in terms of permeate flux and solute rejection. The observed iron rejection, as well
as COD and conductivity, were calculated as follows:
Ri (%) = (1 - (cp,i/cf,i)) x 100 (1)
where cp,i is the concentration of the solute i in the permeate stream, and cf,i the concentration of the solute i in the feed-water tank.
The saturation index (SI) was also calculated following ASTM International (2001). The SI serves to gather information about the tendency of the feed and concentrate streams to lead to the formation of
precipitates on the membrane surface, and is very useful to elaborate corrosion control programs to prevent from scaling on
the membranes (APHA, AWWA, WPCF, 1992; ASTM International, 2001).
The SI can be determined by means of the following expression (ASTM International, 2001; Fariñas Iglesias, 1998):
SI = pH - pHs (2)
where pH is that in the secondary-treated OMW stream, whereas pHs is the solubility pH of the effluent.
For a target feed volume recovery of the feed-stream fixed (Y), the concentration of a component i in the concentrate stream will be (ASTM International, 2001):
[xi]r = [xi]f - (1 - (Y/100)) (3)
where:
[xi]r = concentration (mol/kg) of the i component in the concentrate stream
[xi]f = concentration (mol/kg) of the i component in the feed-stream
Y = feed volume recovery factor (%)
2.4. Analytical proceduresTOP
All the analytical methods were carried out in triplicate with analytical-grade reagents. Chemical oxygen demand (COD), total
phenols (TPh), total suspended solids (TSS), electro-conductivity (EC), pH and particle size distribution (Plus90 nano-sizer,
Brookhaven) were measured following standard methods (Greenberg et al., 2005).
For the measurement of the total iron concentration, all iron ions were reduced to iron ions (II) in a thioglycolate medium
with a derivative of triazine, forming a reddish-purple complex that was determined photometrically at 565 nm (Standard German
methods ISO 8466-1 and German DIN 38402 A51) (Greenberg et al., 2005).
3. RESULTS AND DISCUSIONTOP
3.1. Physcochemical composition of OMW prior to membrane processesTOP
The physicochemical composition of the pretreated OMW is given in Table 2. The goal of the present study was to experimentally evaluate, on a preliminary lab-scale research, the feasibility of a
further treatment of the effluent exiting the Fenton-like process by means of NF or RO technologies for both the recovery
of iron to re-use it as catalyst to reduce its consumption in the Fenton-like reactor, and the removal of the COD and high
EC remaining. The intention was to achieve suitability for re-using the final effluent in the olive oil production process
and therefore closing the loop, and evaluate the efficiency of the pretreatment to preserve the membranes from fouling.
Table 2. Physicochemical composition of secondary-treated OMW.
| Parameters |
Parametric value |
| pH |
7.8 - 8.2 |
| Conductivity, mS/cm |
3.5 - 5.5 |
| Total suspended solids, mg/L |
14 - 16 |
| COD, mg/L |
120.5 - 226.6 |
| Total phenols, μg/L |
390 - 980 |
| Total iron, μg/L |
400 - 1000 |
3.2. Membranes permeate productivityTOP
In first place, the virgin NF and RO membranes’ pure water permeability (m0) was calculated (Table 1) by measuring the permeate flux with MilliQ® water (18 MΩ∙cm) over a range of applied pressures at constant ambient temperature (22 ± 0.1 °C) and turbulent cross-flow
conditions (tangential velocity 5.09 m/s). In the same way, virgin membrane permeability coefficients for the pretreated OMW
(m) were additionally calculated. The linear relationship between the permeate flux-net operating pressures for both pure
water and pre-treated OMW were fitted. The membranes’ pure water permeability coefficients (m0), in L/hm2bar, were found to be equal to 6.1 for AK (RO membrane) and 8.2 for DK (NF membrane). Lower membranes permeability for the
pre-treated OMW (m), in L/hm2bar, was confirmed: 3.9 for AK module RO membrane and 5.3 for DK module NF membrane. These flux gaps can be explained by the
concentration polarization build-up in the boundary region of the membranes.
Next, semi-batch runs were performed with each membrane following the procedure described in section 2.3. Experiments were conducted at different operating pressures: 3, 5 and 8 bar for the AK series RO membrane (the maximum allowable
pressure for this membrane is 8.7 bar, hence a safety margin was adopted), and 5, 7 and 9 bar for the DK series NF membrane
(low pressure framework, in order to obtain comparable results with respect to the RO membrane). Otherwise, turbulent cross-flow
conditions (Reynolds number > 4000) were set, so as to ensure a proper shear rate over the membrane and thus avoid fouling
and concentration polarization phenomena as far as possible (Mott and Untener, 2014). The temperature conditions during the experiments were maintained at ambient temperatures (22 ± 0.1 °C). The influent to the membranes was the effluent from the above described physicochemical secondary treatment (refer to section 2.1), with the characteristics reported in Table 2.
The mean permeate fluxes yielded with each membrane were enhanced linearly upon increasing the operating pressure within the
respective pressure range of each of the selected membranes (Figure 2). Increments of the permeate production were found to fit a linear trend upon increase of the net driving force, namely operating
pressure, for both membranes tested. However, the composite polyamide/polysulfone NF membrane (DK) is able to yield greater
permeate flux productions than those obtained with the RO (AK) one. At an operating pressure of 5 bar, up to 15.9 L/hm2 permeate flux was yielded by the AK (RO) membrane, whereas upon the same operating pressure with the DK (NF) membrane, a
permeate flux equal to 25.3 L/hm2 was measured, which is 37.2% higher. Otherwise, under an operating pressure of 8 bar, about 30.9 L/hm2 permeate flux was obtained with the AK (RO) membrane, while a permeate flux of up to 47.4 L/hm2 was measured upon a similar pressure (9 bar) with the DK (NF) membrane.
|
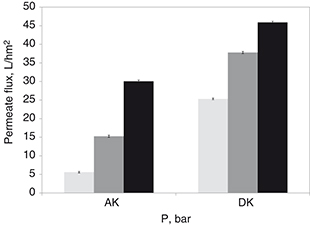 Figure 2. Permeate flux values yielded by the selected membranes: AK series (RO) (■ 3, ■ 5 and ■ 8 bar) and DK series (NF) (■ 5, ■ 7 and ■ 9 bar). Figure 2. Permeate flux values yielded by the selected membranes: AK series (RO) (■ 3, ■ 5 and ■ 8 bar) and DK series (NF) (■ 5, ■ 7 and ■ 9 bar).
|
|
The higher permeate flux obtained with the DK membrane is owed to the nano-porous structure nature of NF membranes, in which
convective transport occurs, where, as in RO membranes, which are widely accepted to be homogeneous surfaces, though exhibiting
imperfections related to their fabrication process, e.g. interfacial polymerization and phase inversion, the solution diffusion
takes place.
The selected NF membrane is a highly productive one, capable of offering very high fluxes at low operating pressures, thereby
it seemed a priori an optimum membrane from the point of view of the optimization of operating costs. The selection of the
proper operating pressure is the key to all membrane processes, in terms of capital and operating expenses, commonly referred
in engineering as “capex” and “opex”. Operating at higher pressure leads to major permeate production, involving smaller membrane
area and shortened working periods, while the other side of the balance implies more energy consumption for the same amount
of influent.
Moreover, another key parameter when projecting membrane treatment plants is the feed volume recovery factor (Y,%). This is
very relevant and and is also connected to membrane fouling. An excessive feed volume recovery would lead to fouling issues
in a shorter period of time, especially in case of batch or semi-batch systems where the bulk becomes increasingly concentrated,
and more irretrievable or irreversible if scaling is a potential type of fouling in the specific system like the one in this
research work (due to the presence of colloidal iron).
Inorganic fouling, in particular that generated by colloidal iron precipitates, has paramount importance as evidenced from
the manufacturers’ recommendations on iron concentrations in feed waters and from the problems frequently encountered in membrane
facilities. Previous studies have warned that a clearly detectable decline in the permeation rate, linear in time, is attained
in the iron concentration range of a few ppm. Given that the solubility of ferric ions at pH = 7 is estimated to be 5.9·10−10 mol/L, even at a total concentration of 1 ppb almost all iron will be in precipitated form (Yiantsios and Karabelas, 2002). Membrane fouling problems have been reported even at lower concentrations for dissolved iron than those recommended by
manufacturers, thus this limit should be considered tentative (Yiantsios and Karabelas, 2002).
In addition, calcium leads to the formation of scaling on the fouled membranes, mainly in the form of calcium carbonate, chlorides
and sulfates. In this regard, it is important to highlight the role of certain ionic species such as calcium ions, which promote
the aggregation of the organic matter by intra and intermolecular bridge formation mechanisms (Madaeni and Samieirad, 2010).
The SI calculated following ASTM International (2001) (see section 2.3) was found to be 0.4 for a Y factor of 80%, increasing up to 0.9 for a Y equal to 90%. This means that higher feed recoveries
are not recommended, given that saturation of the concentrate stream driven back to the bulk tank will lead to deleterious
fouling issues, caused by the promotion of scaling formation given by precipitation of carbonates as well as fouling by colloidal
iron. Hence, a feed volume recovery of Y = 80% should be adopted.
3.3. Membrane rejection performance towards ironTOP
In Table 3, the rejection efficiencies for both membranes with regard to the recovery of iron are reported. A rejection ranging from
95.1 - 99.1% for an operating pressure between 5 - 9 bar was registered for the NF membrane, whereas the RO membrane yielded
a rejection efficiency for iron of 100% disregarding the operating pressure.
Table 3. Iron rejection efficiencies and measured values in permeate streams.
| Membrane |
Op. P, (bar) |
Iron rejection (%) |
Permeate iron (μg/L) |
| DK (NF) |
5 |
95.1 |
19.6 - 49 |
|
7 |
97.5 |
10 - 25 |
|
9 |
99.1 |
3.6 - 9 |
| AK (RO) |
3 |
100 |
n.o. |
|
5 |
100 |
n.o. |
|
8 |
100 |
n.o. |
Table 4. Required membrane area and overdimension of each membrane operation.
| Membrane |
Fouling index α, (L/h2m2bar)
|
OD (%) |
Am required, (m2)
|
Am implemented, (m2)
|
Nmodules |
| NF |
0.0072 |
0.8 |
23.8 |
32 |
2 |
| RO |
0.065 |
10.1 |
32.1 |
64 |
4 |
| Am required: required membrane area; Am implemented: implemented membrane area; OD: membrane area overdimension; Nmodules: number of membrane modules necessary.
|
The rejection behavior of the membrane was further studied and modelized by means of a leaky solution-diffusion model (Jain
and Gupta, 2006):
Ri = PTM ∙ σi / (PTM + βi) (4)
where the rejection of the solute i (Ri) depends primarily on the trans-membrane pressure (PTM) and two parameters, σi which is a reflection coefficient indicating the rejection capability of the membrane (0 < σi < 1) and βi which is a fitting parameter. Accurate predictions of the experimental results were attained by the applied leaky solution-diffusion
model in both cases (coefficient of determination R2 ≥ 0.99).
Results from the fitting of the iron rejection values (Table 3) reveal a σi value equal to 1 and a βi value equal to 0.5 for the NF membrane, whereas for the RO membrane these values were equal to 1 and 0.01, respectively.
These results are in very good agreement with the rejection nature of NF and RO membranes.
These results indicate that both membranes exhibit a good performance for the rejection of the iron (99.1% for the DK series
NF membrane vs. 100% for the AK series RO membrane) in the OMW stream exiting the Fenton-like secondary treatment, thus permitting the recovery
of iron in the concentrate stream in order to recycle it back into the oxidation reactor to reduce catalyst consumption. Finally,
the COD values in the permeate streams were 53.4 - 74.5 vs. 1.4 - 1.9 mg/L for NF and RO, whereas the EC was measured to be 2459 - 2719 vs. 31.1 - 169.1 mg/L for NF and RO, permitting the re-use of the permeate stream for irrigation.
3.4. Membranes plant dimensionTOP
Finally, the required membrane area was calculated on the basis of a daily amount of 10 m3 of OMW treatment need and considering 10 h operation a day (Table 4). The number of the necessary membrane modules (Nmodules, 32 m2 each) was also estimated, as well as the overdesign (OD) of the membrane area. For this purpose, the estimated RO membrane
area (Am) needed for the treatment of the secondary-treated OMW was calculated with the following equation, derived from the boundary
flux theory previously validated by the Stoller and Ochando-Pulido (Ochando and Stoller, 2014; Stoller and Ochando, 2014, 2015):
Am = Vf ∙ (Y⁄100) ∙ (1+(OD⁄100))/Jb (5)
where Am is the required membrane area (m2), Jb is the boundary (steady-state) permeate flux value (L/hm2), Y is the target feed volume recovery set (%) (see section 3.1), Vf is the volume of effluent feedstock to be treated (L/h) and OD is the necessary membrane overdesign (%).
The membrane overdesign was estimated with the following expression successfully used in previous work by the same Authors
(Stoller and Ochando, 2014, 2015):
OD = 100 ∙ (1–(Jb – α ∙ PTM∙tw))/Jb (6)
where tw is the membrane operating period time (h), PTM is the selected net driving pressure (bar) and α is the long-term fouling index (L/h2m2bar). A thorough description of these equations and calculations can be found in Stoller and Ochando (2015).
An Am equal to 23.8 m2 was estimated for the NF membrane whereas 32.1 m2 for the RO one. Applying a conservative safety margin of 10%, final Am of 26.3 m2 and 35.2 m2 would be necessary, respectively. This means one NF membrane module (plus another in parallel which works alternatively when
the cleaning protocol is performed on the used NF membrane), where two RO modules are needed (plus 2 additional in parallel,
operating alternatively during the performance of the cleaning protocol on the used RO membranes).
4. CONCLUSIONSTOP
In this work, two different membranes, one nano-filtration (NF) and a low-pressure reverse osmosis (RO), are examined with
a double aim: the main one is the recovery of the iron used as catalyst in the former Fenton-like secondary treatment of OMW.
The results indicate that both membranes exhibit a good performance towards the rejection of iron (99.1% for the DK series
NF membrane vs. 100% for the AK series RO membrane) in the OMW stream after the Fenton-like secondary treatment. This would permit the recovery
of iron in the concentrate stream in order to recycle it back into the oxidation reactor to reduce the consumption of catalyst.
Finally, the permeate streams could be re-used for irrigation.
However, the productivity of the selected NF membrane increases upon lowing operating pressures, about 30.9 L/hm2 under at 8 bar with the RO membrane while 38.2 - 47.4 L/hm2 upon 8- 9 bar with the NF membrane. Moreover, a sensibly lower fouling index was measured on the NF membrane (0.0072 in contrast
with 0.065), which ensures major steady-state performance on this membrane and longer service lifetime. Furthermore, this
also results in a lower required membrane area, which is 4 modules in the case of RO in contrast with 2 modules for NF.
ACKNOWLEDGMENTSTOP
The Spanish Ministry of Science and Innovation is gratefully acknowledged for having funded the projects CTQ2007-66178 and
CTQ2010-21411, as well as the University of Granada.
REFERENCESTOP
| ○ |
Akar T, Tosun I, Kaynak Z, Ozkara E, Yeni O, Sahin E N, Akar S T. 2009. An attractive agro-industrial by-product in environmental
cleanup: Dye biosorption potential of untreated olive pomace. J. Hazard. Mater. 166, 1217-1225. http://dx.doi.org/10.1016/j.jhazmat.2008.12.029 |
| ○ |
Akdemir EO, Ozer A. 2009. Investigation of two ultrafiltration membranes for treatment of olive oil mill wastewater. Desalination 249, 660-666. http://dx.doi.org/10.1016/j.desal.2008.06.035 |
| ○ |
Aktas ES, Imre S, Esroy L. 2001. Characterization and lime treatment of olive mill wastewater. Water Res. 35, 2336-2340.
|
| ○ |
Al-Malah K, Azzam MOJ, Abu-Lail NI. 2000. Olive mills effluent (OME) wastewater post-treatment using activated clay. Sep. Purif. Technol. 20, 225-234. http://dx.doi.org/10.1016/S1383-5866(00)00114-3 |
| ○ |
Annesini M, Gironi F. 1991. Olive oil mill effluent: ageing effects on evaporation behavior. Water Research, 25, 1157-1960. http://dx.doi.org/10.1016/0043-1354(91)90210-H |
| ○ |
APHA, AWWA, WPCF. 1992. Standard Methods for water and wastewater analysis. Díaz de Santos, p. 1816, ISBN: 84-7978-031-2,
Madrid.
|
| ○ |
ASTM International D 4582 - 91, 2001. Standard Practice for Calculation and Adjustment of the Stiff and Davis Stability Index
for Reverse Osmosis.
|
| ○ |
Beltrán J, Torregrosa J, García J, Domínguez JR. 2000. Ozone treatment of olive mill wastewater. Grasas Aceites 51, 32-46. http://dx.doi.org/10.3989/gya.2000.v51.i5.428 |
| ○ |
Baccar R, Bouzid J, Feki M, Montiel A. 2009. Preparation of activated carbon from Tunisian olive-waste cakes and its application
for adsorption of heavy metal ions. J. Hazard. Mater. 162, 1522-1529. http://dx.doi.org/10.1016/j.jhazmat.2008.06.041 |
| ○ |
Borja R, Raposo F, Rincón B. 2006. Treatment technologies of liquid and solid wastes from two-phase olive oil mills. Grasas Aceites 57, 32-46.
|
| ○ |
Cegarra J, Paredes C, Roig A, Bernal MP, García D. 1996. Use of olive mill wastewater compost for crop production. Int. Biodet. Biodegrad. 38, 193-203. http://dx.doi.org/10.1016/S0964-8305(96)00051-0 |
| ○ |
Coskun T, Debik E, Demir N M. 2010. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 259, 65-70. http://dx.doi.org/10.1016/j.desal.2010.04.034 |
| ○ |
Ena A, Carlozzi P, Pushparaj B, Paperi R, Carnevale S, Sacchi A. 2007. Ability of the aquatic fern Azolla to remove chemical
oxygen demand and polyphenols from olive mill wastewater. Grasas Aceites 58, 32-46.
|
| ○ |
Fariñas Iglesias M. 1998. Ósmosis inversa: fundamentos, tecnología y aplicaciones. Ed. McGraw-Hill. |
| ○ |
Garcia-Castello E, Cassano A, Criscuoli A, Conidi C, Drioli E. 2010. Recovery and concentration of polyphenols from olive
mill wastewaters by integrated membrane system. Water Res. 44, 3883-3892. http://dx.doi.org/10.1016/j.watres.2010.05.005 |
| ○ |
Garrido Hoyos SE, Martínez Nieto L, Camacho Rubio F, Ramos Cormenzana A. 2002. Kinetics of aerobic treatment of olive-mill
wastewater (OMW) with Aspergillus terreus. Process Biochem. 37, 1169-1176. http://dx.doi.org/10.1016/S0032-9592(01)00332-6 |
| ○ |
Grafias P, Xekoukoulotakis NP, Mantzavinos D, Diamadopoulos E. 2010. Pilot treatment of olive pomace leachate by vertical-flow
constructed wetland and electrochemical oxidation: an efficient hybrid process. Water Research 44, 2773-2780. http://dx.doi.org/10.1016/j.watres.2010.02.015 |
| ○ |
Greenberg AE, Clesceri LS, Eaton AD. 2005. Standard Methods for the Examination of Water and Wastewater, APHA/AWWA/WEF, 22th
ed., Washington DC. Cabs.
|
| ○ |
Haddadin M S Y, Haddadin J, Arabiyat O I, Hattar B. 2009. Biological conversion of olive pomace into compost by using Trichoderma harzianum and Phanerochaete chrysosporium. Biores. Tech. 100, 4773-4782. http://dx.doi.org/10.1016/j.biortech.2009.04.047 |
| ○ |
Hodaifa G, Ochando-Pulido JM, Rodriguez-Vives S, Martínez-Férez A. 2013a. Optimization of continuous reactor at pilot scale
for olive-oil mill wastewater treatment by Fenton-like process. Chem. Eng. J. 220, 117-124. http://dx.doi.org/10.1016/j.cej.2013.01.065 |
| ○ |
Hodaifa G, Eugenia-Sánchez M, Sánchez S. 2008. Use of industrial wastewater from olive-oil extraction for biomass production
of Scenedesmus obliquus. Bioresour. Technol. 99, 1111-1117. http://dx.doi.org/10.1016/j.biortech.2007.02.020 |
| ○ |
Hodaifa G, Ochando-Pulido JM, Ben-Driss-Alami S, Rodriguez-Vives S, Martínez-Férez A. 2013b. Kinetic and thermodynamic parameters
of iron adsorption onto olive stones. Ind. Crops Prod. 49, 526-534. http://dx.doi.org/10.1016/j.indcrop.2013.05.039 |
| ○ |
Inan H, Dimoglo A, Şimşek H, Karpuzcu M. 2004. Olive oil mill wastewater treatment by means of electro-coagulation. Sep. Purif. Technol. 36, 23-31. http://dx.doi.org/10.1016/S1383-5866(03)00148-5 |
| ○ |
Jain S, Gupta SK, 2004. Analysis of modified surface force pore flow model with concentration polarization and comparison
with Spiegler-Kedem model in reverse osmosis systems. J. Membr. Sci. 232, 45-62. http://dx.doi.org/10.1016/j.memsci.2003.11.021 |
| ○ |
Lafi WK, Shannak B, Al-Shannag M, Al-Anber Z, Al-Hasan M. 2009. Treatment of olive mill wastewater by combined advanced oxidation
and biodegradation. Separ. Purif. Technol. 70, 141-146. http://dx.doi.org/10.1016/j.seppur.2009.09.008 |
| ○ |
Madaeni SS, Samieirad S. 2010. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 257, 80-86. http://dx.doi.org/10.1016/j.desal.2010.03.002 |
| ○ |
Martínez Nieto L, Ben Driss Alami S, Hodaifa G, Faur C, Rodríguez Vives S, Giménez Casares JA, Ochando J. 2010. Adsorption
of iron on crude olive stones. Ind. Crop. Prod. 32, 467-471. http://dx.doi.org/10.1016/j.indcrop.2010.06.017 |
| ○ |
Martínez Nieto L, Hodaifa G, Rodríguez Vives S, Giménez Casares JA, Ochando J. 2011a. Flocculation-sedimentation combined
with chemical oxidation process. Clean - Soil, air, water 39, 949-955. http://dx.doi.org/10.1002/clen.201000594 |
| ○ |
Martínez Nieto L, Hodaifa G, Rodríguez Vives S, Giménez Casares JA, Ochando J. 2011b. Degradation of organic matter in olive
oil mill wastewater through homogeneous Fenton-like reaction. Chem. Eng. J. 173, 503-510. http://dx.doi.org/10.1016/j.cej.2011.08.022 |
| ○ |
Marques IP. 2001. Anaerobic digestion treatment of olive mill wastewater for effluent re-use in irrigation. Desalination 137, 233-239. http://dx.doi.org/10.1016/S0011-9164(01)00224-7 |
| ○ |
Mott R L, Untener J A, Applied Fluid Mechanics, 7th edition, University of Dayton, 2014. |
| ○ |
Ochando-Pulido JM, Rodriguez-Vives S, Martínez-Férez A. 2012a. The effect of permeate recirculation on the depuration of pretreated
olive mill wastewater through reverse osmosis membranes. Desalination 286, 145-154. http://dx.doi.org/10.1016/j.desal.2011.10.041 |
| ○ |
Ochando-Pulido JM, Hodaifa G, Rodriguez-Vives S, Martínez-Férez A. 2012b. Impacts of operating conditions on reverse osmosis
performance of pretreated olive mill wastewater. Water Res. 46, 4621-4632. http://dx.doi.org/10.1016/j.watres.2012.06.026 |
| ○ |
Ochando-Pulido JM, Hodaifa G, Victor-Ortega MD, Rodriguez-Vives S, Martínez-Férez A, 2013a. Effective treatment of olive mill
effluents from two-phase and three-phase extraction processes by batch membranes in series operation upon threshold conditions.
J. Hazard. Mater. 263, 168-176. http://dx.doi.org/10.1016/j.jhazmat.2013.03.041 |
| ○ |
Ochando-Pulido JM, Hodaifa G, Victor-Ortega MD, Rodriguez-Vives S, Martínez-Férez A, 2013b. Reuse of olive mill effluents
from two-phase extraction process by integrated advanced oxidation and reverse osmosis treatment, J. Hazard. Mater. 263, 158-67. http://dx.doi.org/10.1016/j.jhazmat.2013.07.015 |
| ○ |
Ochando-Pulido JM, Hodaifa G, Victor-Ortega MD, Martínez-Férez A, 2014. A novel photocatalyst with ferromagnetic core used
for the treatment of olive oil mill effluents from two-phase production process. The Scientific World Journal 2014. http://dx.doi.org/10.1155/2013/196470 |
| ○ |
Ochando-Pulido J.M., Stoller M, 2014. Boundary flux optimization of a nanofiltration membrane module used for the treatment
of olive mill wastewater from a two-phase extraction process. Separ. Purif. Technol. 130, 124-131. http://dx.doi.org/10.1016/j.seppur.2014.04.035 |
| ○ |
Papadimitriou EK, Chatjipavlidis I, Balis C. 1997. Application of composting to olive mill wastewater treatment. Environ. Technol. 18, 101-107. http://dx.doi.org/10.1080/09593331808616517 |
| ○ |
Paraskeva P, Diamadopoulos E. 2006. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 81, 475-485. http://dx.doi.org/10.1002/jctb.1553 |
| ○ |
Paraskeva C A, Papadakis V G, Tsarouchi E, Kanellopoulou D G, Koutsoukos P G. 2007. Membrane processing for olive mill wastewater
fractionation. Desalination 213, 218-229. http://dx.doi.org/10.1016/j.desal.2006.04.087 |
| ○ |
Russo C. 2007. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic
substances from vegetation waters (VW). J. Membr. Sci. 288, 239-246. http://dx.doi.org/10.1016/j.memsci.2006.11.020 |
| ○ |
Sacco O, Stoller M, Vaiano V, Ciambelli P, Chianese A, Sannino D. 2012. Photocatalytic degradation of organic dyes under visible
light on n-doped photocatalysts. Int. J. Photoenergy 2012. http://dx.doi.org/10.1155/2012/626759 |
| ○ |
Sarika R, Kalogerakis N, Mantzavinos D. 2005. Treatment of olive mill effluents. Part II. Complete removal of solids by direct
flocculation with poly-electrolytes. Environ. Int. 31, 297-304. http://dx.doi.org/10.1016/j.envint.2004.10.006 |
| ○ |
Stasinakis A S, Elia I, Petalas A V, Halvadakis C P. 2008. Removal of total phenols from olive-mill wastewater using an agricultural
by-product, olive pomace. J. Hazard. Mater. 160, 408-413. http://dx.doi.org/10.1016/j.jhazmat.2008.03.012 |
| ○ |
Stoller M. 2008. Technical optimization of a dual ultrafiltration and nanofiltration pilot plant in batch operation by means
of the critical flux theory: a case study. Chem. Eng. Process. 47, 1165-1170. http://dx.doi.org/10.1016/j.cep.2007.07.012 |
| ○ |
Stoller M. 2009. On the effect of flocculation as pretreatment process and particle size distribution for membrane fouling
reduction. Desalination 240, 209-217. http://dx.doi.org/10.1016/j.desal.2007.12.042 |
| ○ |
Stoller M. 2011. Effective fouling inhibition by critical flux based optimization methods on a NF membrane module for olive
mill wastewater treatment. Chem. Eng. J. 168, 1140-1148. http://dx.doi.org/10.1016/j.cej.2011.01.098 |
| ○ |
Stoller M, Ochando-Pulido JM. 2012. Going from a critical flux concept to a threshold flux concept on membrane processes treating
olive mill wastewater streams. Procedia Eng. 44, 607-608. http://dx.doi.org/10.1016/j.proeng.2012.08.500 |
| ○ |
Stoller M, Ochando-Pulido J.M., 2014. About merging threshold and critical flux concepts into a single one: the boundary flux.
The Scientific World J. 2014, 656101. http://dx.doi.org/10.1155/2014/656101 |
| ○ |
Stoller M, Ochando-Pulido J.M., 2015. The boundary flux handbook: A comprehensive database of critical and threshold flux
values for membrane practitioners, Amsterdam (Netherlands), Elsevier.
|
| ○ |
Stoller M, Ochando-Pulido JM, di Palma L, Martínez-Férez A. 2015. Membrane process enhancement of 2-phase and 3-phase olive
mill wastewater treatment plants by photocatalysis with magnetic-core titanium dioxide nanoparticles. J. Ind. & Eng. Chem. In press, 2015. http://dx.doi.org/10.1016/j.jiec.2015.05.015 |
| ○ |
Tekin A R, Coşkun Dalgıç A. 2000. Biogas production from olive pomace. Resour. Conserv. Recy. 30, 301-313. http://dx.doi.org/10.1016/S0921-3449(00)00067-7 |
| ○ |
Tezcan Ü, Uğur S, Koparal AS, Öğütveren ÜB. 2006. Electrocoagulation of olive mill wastewaters. Sep. Purif. Technol. 52, 136-141. http://dx.doi.org/10.1016/j.seppur.2006.03.029 |
| ○ |
Turano E, Curcio S, De Paola M G, Calabrò V, Iorio G. 2002. An integrated centrifugation–ultrafiltration system in the treatment
of olive mill wastewater. J. Membr. Sci. 206, 519-531. http://dx.doi.org/10.1016/S0376-7388(02)00369-1 |
| ○ |
Vincent-Vela MC, Cuartas-Uribe B, Álvarez-Blanco S, Lora-García J. 2011. Analysis of fouling resistances under dynamic membrane
filtration. Chem. Eng. Process. 50, 404–408. http://dx.doi.org/10.1016/j.cep.2011.02.010 |
| ○ |
Yiantsios S G, Karabelas A J. 2002. An assessment of the Silt Density Index based on RO membrane colloidal fouling experiments
with iron oxide particles. Desalination 15, 229-238. http://dx.doi.org/10.1016/S0011-9164(02)01015-9 |
Figure 1. Flow diagram of the bench-scale RO unit. V1, V2: emptying valves (pump inlet and outlet respectively); V3, V4: pressure regulating
valves for module 2 and 1 respectively; V5: venting valve for module M1; V6: three-way valve to select desired working membrane
module; V7: magnetic valve for cooling jacket inlet; M1: flat-sheet membrane module; M2: spiral-wounded module; P: feedstock
pump; FT: feedstock tank; PISH01, PISH02: pressure gauges; TICSH01: temperature gauge.