Fatty acid composition of the pollen lipids of Cycas revoluta Thunb
R.A. Sidorov*, E.I. Kuznetsova, V.P. Pchelkin, A.V. Zhukov, E.N. Gorshkova and V.D. Tsydendambaev
Laboratory of Lipid Metabolism, K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, Botanicheskaya
str., 35, Moscow, 127276, Russia.
*Corresponding author: roman.sidorov@mail.ru
| |
SUMMARY
The fatty acid (FA) composition of total extractable and non extractable with chloroform lipids of C. revoluta pollen was determined. Among other minor FAs, unusual Δ5 polymethylene-interrupted FA, Δ5, 11-octadecadienoic acid was found. This FA was found in the seed lipids of C. revoluta earlier, but it was discovered for the first time in pollen lipids.
KEYWORDS: Cycas revoluta;
Fatty acids;
Gymnosperm species;
Pollen;
Sago palm;
Δ5-fatty acids
|
| |
RESUMEN
Composición en ácidos grasos de los lípidos del polen de palmeras Cycas revoluta. Se determinó la composición en ácidos grasos (AG) de los lípidos totales extraíbles y no extraíbles con cloroformo del polen
de la palmera C. revoluta. Entre otros ácidos grasos menores se encontró un AG Δ5 inusual, el ácido octadecadienoico, Δ5,11-polimetilen-interrumpido.
Este AG ya fue descrito en los lípidos de semillas de C. revoluta, pero en los lípidos del polen es la primera vez que se describen.
PALABRAS CLAVE: Ácidos grasos;
Ácidos grasos Δ5-;
Cycas revoluta;
Especies de gimnospermas;
Palma de sagú;
Polen
|
1. INTRODUCTIONTOP
Cycas L. (false sago palm) is an ancient genus of Gymnosperms, a group of more than 90 species, the only genus of the family Cycadaceae.
It is expected that Cycadaceae are the earliest seed plants, like the ginkgo, descending from long extinct seed ferns (Laubenfels and Adema, 1998). Cycadaceae are pollinated by the wind similar to other dioecious plants. For decorative purposes only a few Cycas species are used, among which the most popular is Cycas revoluta, originally from Southeast Asia. The lifetime of C. revoluta reaches more than 100 years, greenhouse specimens can bloom only a few times during growth (Hill et al., 2004; Kramer and Green, 1990). However this phenomenon is hardly to be called flowering as such, because Cycas plants belong to the varieties of Gymnosperms, which have no fruits and no true flowers. The specimen of C. revoluta, which has grown in greenhous of the K.A. Timiryazev Institute of Plant Physiology for several decades, has bloomed this
year for the first time. Since the fatty acid (FA) composition of Cycas lipids has been investigated only in leaves (Mongrand et al., 2001) and seeds (Takagi and Itabashi, 1982), and taking into account the extreme rarity of the flowering of this plant, we decided to study the FA composition of the
total lipids of its pollen (microspores).
2. MATERIALS AND METHODSTOP
2.1. Plant material and extraction of lipidsTOP
Pollen grains were collected by means of glass rods from the microstrobile of a blooming male plant of the Cycas revoluta Thunb. growing in the greenhouse of the K.A. Timiryazev Institute of Plant Physiology of RAS. Lipids were extracted from
the plant material (500 mg) with 100 mL of purified chloroform for 30 min, the extract was filtered out and the solvent was
removed with a vacuum evaporator. The FAs of the lipids of the dry residue were converted into methyl esters by direct transesterification
with 5 mL of a 10% metanolic solution of acetyl chloride (Sidorov et al., 2014). The FAs of non extractable residue were obtained by direct saponification with 10 mL of a 6% solution of potassium hydroxide
in 80% aqueous methanol followed by extraction of free FAs with hexane and their subsequent conversion into methyl esters
by a common procedure (Sidorov et al., 2014). All the solvents contained 0.001% of butylated hydroxytoluene as antioxidant.
2.2. Synthesis of 4’4’-dimethyloxazoline derivatives of fatty acidsTOP
In order to determine the double bond position of unidentified FAs, we used mass-spectrometry of their 4’4’-dimethyloxazoline
derivatives also known as DMOXes, which we synthesized following a common procedure with slight modifications. We added 200
μL of oxalyl chloride to 5 mg of free fatty acids obtained by saponification of plant material (see section 2.1), then placed
a screw cap vial with this mixture into the 50 ml centrifuge tube filled with anhydrous sodium sulfate. The tube was left
for one hour in the water bath heated to 45 °C with the subsequent evaporation of oxalyl chloride under a stream of argon.
Then we added 300 μL of a 20% solution of amino-2-propanol in dichloromethane to the chloroanhydrides of the FFAs and left
the vial for one hour at the room temperature. The solvent was evaporated under a stream of argon. Afterwards we added 300
μL of trifluoroacetic anhydride to the dry residue and kept the vial at 45 °C for 1 hour. Finally we removed the excess solvent
in a stream of argon, added 100 μL of distilled water and 200 μL of hexane, shook the vial vigorously, collected the hexane
layer into a new vial and dried it over anhydrous sodium sulfate for 20 min (Christie, 2012b). The DMOXes thus obtained were immediately analyzed by GC–MS (see section 2.3).
2.3. Analysis of fatty acidsTOP
The qualitative and quantitative FA compositions in the lipid preparations were determined by GC–MS using an internal standard
technique; heptadecanoic acid methyl ester was an internal standard (Sidorov et al., 2014). Fatty acid methyl esters were analyzed by GC–MS using an Agilent 7890A GC device fitted with a capillary column (DB-23,
60 m × 0.25 mm) containing a grafted (50% cyanopropyl)-methylpolysiloxane polar liquid phase as a 0.25 μm-thick film. The
FAMEs were separated under the following conditions: operational gas (helium) flow in the column at 1 mL/min, sample volume,
1 μL; flow split ratio, 1:10; evaporator temperature, 260 °C. The oven temperature program was as follows: from 130 to 170
°C at 6.5 °C/min, to 215 °C at 2.75 °C/min (25 min at this temperature), to 240 °C at 40 °C/min, and 50 min at 240 °C, operational
temperature of the mass selective detector (Agilent 5975C MSD), 240 °C; ionization energy, 70 eV. For identifying individual
FAME species and calculating their concentrations in the mixture, a NIST research library v. 2.0 and MSD Chem Station E.02.00.493
software were used (Sidorov et al., 2014). All experiments were performed with three replicates.
3. RESULTS AND DISCUSSIONTOP
The FA compositions of total extractable and non extractable with CHCl3 (mainly neutral and polar) lipids of C. revoluta pollen are presented in Table 1. One can see that the diversity of the FA composition was higher in the extractable with CHCl3 pollen lipids (28 individual FAs species) than in the non extractable ones (21 FAs species). Major FAs in both pollen lipid
fractions were palmitic (16:0), oleic (Δ9-18:1) and linoleic (Δ9,12-18:2) acids; stearic (18:0) and α-linolenic (Δ9,12,15-18:3)
acids were also present in appreciable quantities. In both the extractable and non extractable with CHCl3 lipids of C. revoluta pollen in minor quantities several unusual FAs namely Δ7-18:1, Δ9,11-18:2, Δ5,9-18:2 (taxoleic), Δ5,9,12-18:3 (pinolenic),
and Δ5,11,14-20:3 (sciadonic) were found as well as several FAs with very long chains (C≥ 20).
Table 1. Fatty acids composition of extractable and non extractable with CHCl3 lipids of C. revoluta pollen, mas.-% of total FAsa
| Fatty acid |
ECL |
Extractable lipids |
Non extractable lipids |
| 14:0 |
14.00 |
0.4±0.0 |
0.6±0.2 |
| 15:0 |
15.00 |
0.2±0.0 |
0.4±0.0 |
| 16:0 |
16.00 |
25.2±0.4 |
30.9±1.0 |
| Δ7-16:1 |
16.20 |
0.3±0.0 |
0.4±0.0 |
| Δ9-16:1 |
16.29 |
0.3±0.0 |
0.2±0.0 |
| Δ7,10-16:2 |
16,68 |
0.1±0.0 |
−b
|
| Δ7,10,13-16:3 |
17.27 |
0.1±0.0 |
− |
| 17:0 |
17.00 |
0.3±0.0 |
1.0±0.0 |
| 18:0 |
18.00 |
2.4±0.1 |
6.3±0.1 |
| Δ7-18:1 |
18.15 |
0.1±0.0 |
0.6±0.0 |
| Δ9-18:1 |
18.29 |
27.7±0.1 |
18.6±0.3 |
| Δ11-18:1 |
18.33 |
1.0±0.0 |
1.2±0.0 |
| Δ5,9-18:2 |
18.41 |
0.8±0.0 |
0.4±0.0 |
| X |
18.47 |
0.8±0.0 |
− |
| Δ9,11-18:2 |
18.65 |
0.1±0.0 |
− |
| Δ9,12-18:2 |
18.79 |
30.7± 0.5 |
28.4±0.1 |
| Δ5,9,12-18:3 |
18.90 |
0.7±0.0 |
− |
| Δ9,12,15-18:3 |
19.32 |
3.7±0.2 |
3.1±0.0 |
| 19:0 |
19.00 |
0.1±0.0 |
0.1±0.1 |
| 20:0 |
20.00 |
0.1±0.1 |
1.3±0.2 |
| Δ11-20:1 |
20.25 |
0.4±0.0 |
− |
| Δ8,11-20:2 |
20.40 |
0.4±0.0 |
− |
| Δ11,14-20:2 |
20.75 |
0.1±0.0 |
0.1±0.1 |
| Δ5,11,14-20:3 |
20.91 |
2.6±0.1 |
1.9±0.1 |
| 21:0 |
21.00 |
0.1±0.0 |
0.1±0.1 |
| 22:0 |
22.00 |
0.7±0.0 |
2.9±0.4 |
| 23:0 |
23.00 |
0.1±0.0 |
0.1±0.1 |
| 24:0 |
24.00 |
0.4±0.0 |
1.3±0.3 |
| ameans ± SD. |
b“−” − not detected. |
During the analysis of the CHCl3-extractable lipids of C. revoluta pollen, we drew the attention to a minor FA (RT of its methyl ester was equal to 21.86 min, RRT relative to C18:0=1.063 and calculated ECL equal to 18.47). An automated search of mass spectra libraries NIST08 and Wiley did not result in
the identification of the monitoring component because of more than 90% overlap with library mass spectrum of the methyl ester
of the taxoleic acid (Δ5,9-18:2). However, since the chromatographic parameters calculated for this peak differed from the
peak of the latter, we decided to identify unknown FA (X, presumably the x,y-18:2) using the mass spectrometry of its 4,4-alkenyl-dimethyloxazoline (DMOX) derivative.
The mass spectrum of the DMOX derivative of unknown FA from pollen lipids of C. revoluta is presented in Figure 1. Molecular ion M+ with m/z = 333 and its fragmentation profile indicates the location of an octadecadienoic acid with ethylene bonds in an unusual place.
The existence of a fragmentary ion with an odd value m/z=153 (marked with asterisk) is an important diagnostic sign indicating the position of the double bond at the 5th carbon atom
of the FA residue. A characteristic couple of fragmentary ions with a difference of 26 a.m.u. for the Δ5 double bond location with m/z 140 and 166 (Christie, 2012a) is also present in the mass spectrum. From Figure 1 one can see that the relative intensity of ions with greater masses is significantly lower than the m/z=153 ion intensity, except for fragmentary ion with m/z=180, which usually indicates that the double bonds in the FA acyl are separated by more than one −CH2− group. The presence of this ion is characteristic for the FA with so-called polymethylene-interrupted bis-oriented double bonds (Christie, 2012a; Wolff and Christie, 2002). The character of fragmentation, leading a pair of fragmentary ions with m/z=153 and 180 to be generated, is shown in the Figure 1.
|
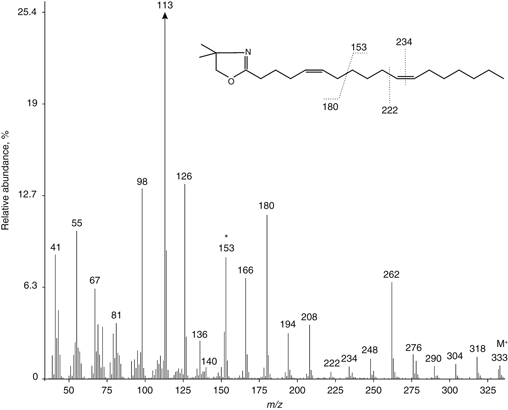 Figure 1. Mass spectrum of the DMOX derivative of unknown FA (X, see Table) from pollen lipids of C. revoluta. Intensities of the remaining fragmentary ions are brought relatively to ion with m/z=113, one of the characteristic ions of FA DMOX derivatives, taken as 100%. Figure 1. Mass spectrum of the DMOX derivative of unknown FA (X, see Table) from pollen lipids of C. revoluta. Intensities of the remaining fragmentary ions are brought relatively to ion with m/z=113, one of the characteristic ions of FA DMOX derivatives, taken as 100%.
|
|
This assumption is confirmed by the fact that the mass-spectra of DMOX derivatives of other FAs with the same value of M+, for example, Δ6- or Δ9-octadecenoic, as well as the Δ8,9-methylene-9-heptadecenoic or Δ13-cyclopentyl-2-enyl-tridecanoic
acids, are substantially different in their pattern of fragmentation (Christie, 2012b). Starting the ion with m/z=166, a series of fragmentary ions, differing in 14 a.m.u. follows. This is typical for fragmentation under the sequential detachment of –CH2– groups from FA acyl. This order is broken by ions with m/z 222 and 234, where the difference in 12 a.m.u. indicates the presence of an ethylene bond at the 11th carbon atom of FA acyl (a second pair of ions, important to determine the exact location of the double bond must differed
by 26 a.m.u., in this case, it is ions with m/z 222 and 248). Thus, it can be concluded that the unusual fatty acid found in the total lipids of C. revoluta pollen is nothing other than Δ5,11-octadecadienoic acid, belonging to a group of bis-polymethylene-interrupted FAs (UPIFAs).
Earlier, the FA composition of C. revoluta lipids was studied in the leaves (Mongrand et al., 2001) and seeds (Takagi, Itabashi, 1982) of this plant. However, to the best of our knowledge, the FA composition of C. revoluta pollen lipids has never been investigated. Leaf lipids were found to contain 21 C14-C22 FAs (Mongrand et al., 2001), and the seed ones more than 23 C13-C22 FAs (Takagi and Itabashi, 1982). The major FAs of leaf lipids were 16:0, Δ9,12-18:2 and Δ9,12,15-18:3 acids and 16:0, Δ9-18:1 and Δ9,12-18:2 acids predominated in the seed ones. Both leaf and seed lipids of C. revoluta contained small quantities of several C18 and C20 Δ5-UPIFA, however, besides pollen, Δ5,11-18:2 FA was found only in the latter (Takagi and Itabashi, 1982). The composition of Δ5-UPIFA in the leaves and seeds was more diverse than in pollen lipids (6, 6, and 4 individual FA species, respectively). In particular, the leaves and seeds contained Δ5,9,12,15-18:4 and Δ5,11,14,17-20:4 FAs, which were absent in
the pollen.
Takagi and Itabashi have studied the FA composition of the seed lipids of 21 species of Gymnosperms, but besides C. revoluta Δ5,11-18:2 FA was identified only in the seed lipids of G. biloba, Ephedra sinica and in Podocarpus macrophylla (Takagi and Itabashi, 1982). According to data of Mongrand et al., who studied the FA composition of the leaf lipids of 137 species of Gymnosperms belonging to 14 families, including the leaves of C. revoluta, lipids of the latter, as the overwhelming majority of other Gymnosperms studied, along with conventional C14-C22 FAs in small quantities contained anteiso-17:0 FA, taxoleic, coniferonic (Δ5,9,12,15-18:4), pinolenic, Δ5,11-20:2, sciadonic and uniperonic (Δ5,11,14,17-20:4) acids
(Mongrand et al., 2001). However, in the leaves of all the Gymnosperm species studied by Mongrand et al. Δ5,11-18:2 FA was not detected.
4. CONCLUSIONSTOP
To the best of our knowledge, Δ5,11-octadecadienoic acid was found for the first time in Dictyostelium discoideum lipids by Davidoff and Korn (Davidoff and Korn, 1962). Soon afterwards, Gellerman and Schlenk discovered this FA in the seed and leaf lipids of Ginkgo biloba (Gellerman and Schlenk, 1963) but unambiguously the structure of this FA has been established by Wolff and coworkers, who found it in Ephedra as well as in G. biloba seed oils and identified by it GC-MS of its nicotinyl and DMOX derivatives (Wolff et al., 1999). Wolff and coworkers proposed a trivial name − ephedrinic acid for Δ5,11–18:2 FA, and suggested several possible pathways
for its biosynthesis (Wolff and Christie, 2002; Wolff et al., 1999). The first of the proposed pathways was connected with the C2-elongation of palmitoleic acid with the formation of cis-vaccenic acid and its subsequent Δ5-desaturation. The authors also suggested that there are two Δ5-desaturases, strictly
specific to oleat- and cis-vaccenate (Wolff et al., 1999). Under the second probable pathway of the Δ5-UPIFA biosynthesis the crucial role is played not by the positions of the double
bonds in the FA acyl, but by the carbon chain length. This hypothesis was based on the results of the investigation of the
FA composition of numerous species of Gymnosperms (Wolff and Christie, 2002; Wolff et al., 2000).
Wolff and coworkers also supposed the simultaneous existence of two Δ5-desaturases specific for C18 and C20 unsaturated FAs or for the Δ9 and Δ11 positions of the first double bond, respectively, because along with ephedrinic acid,
the Δ5,11-eicosadienoic (keteeleronic) acid often appeared (Wolff and Christie, 2002; Wolff et al., 1999; Wolff et al., 2000). Nevertheless, the question of the biosynthesis of unusual Δ5-acids in Gymnosperms remains unanswered.
REFERENCESTOP
| ○ |
Davidoff F, Korn ED. 1962. Lipids of Dictyostelium discoideum: Phospholipid composition and the presence of two new fatty acids: cis,cis-5,11-octadecadienoic and cis,cis-5,9-hexadecadienoic acids. Biochem. Biophys. Res. Commun. 9, 54−58.
|
| ○ |
Christie WW. 2012a. Dienoic Fatty Acids. Mass Spectra of DMOX Derivatives: Methylene-Interrupted Dienoic Fatty Acids. http://lipidlibrary.aocs.org/content.cfm?ItemNumber=39500
|
| ○ |
Christie WW. 2012b. 4,4-Dimethyloxazoline (DMOX) Derivatives of Fatty Acids.
|
| ○ |
Gellerman JL, Schlenk H. 1963. A Group of Fatty Acids with Tetramethylene Interruption in the Double Bond System, Experientia 19, 522–523. http://dx.doi.org/10.1007/BF02150889
|
| ○ |
Hill KD, Stevenson DW, Osborne R. 2004. The World List of Cycads. The Bot. Rev. 70, 274–298.
|
| ○ |
Kramer KU, Green PS, Götz E. 1990. Pteridophytes and Gymnosperms. Berlin: Springer-Verlag. p. 370. http://dx.doi.org/10.1007/978-3-662-02604-5
|
| ○ |
Laubenfels DJ, Adema F. 1998. A taxonomic revision of the genera Cycas and Epicycas Gen. Nov. (Cycadaceae). Blumea 43, 351–400.
|
| ○ |
Mongrand S, Badoc A, Patouille B, Lacomblez Ch, Chavent M, Cassagne C, Bessoule J-J. 2001. Taxonomy of gymnospermae: multivariate
analyses of leaf fatty acid composition. Phytochemistry 58, 101–115. http://dx.doi.org/10.1016/S0031-9422(01)00139-X
|
| ○ |
Takagi T, Itabashi Y. 1982. cis-5 Olefinic Unusual Fatty Acids in Seed Lipids of Gymnospermae and Their Distribution in Triacylglycerols. Lipids 17, 716–723. http://dx.doi.org/10.1007/BF02534657
|
| ○ |
Sidorov RA, Zhukov AV, Pchelkin VP, Vereshchagin AG, Tsydendambaev VD. (2014) Content and Fatty Acid Composition of Neutral
Acylglycerols in Euonymus Fruits J. Am. Oil Chem. Soc. 91, 805–814. http://dx.doi.org/10.1007/s11746-014-2425-2
|
| ○ |
Wolff RL, Christie WW. 2002. Structures, practical sources (gymnosperm seeds), gas-liquid chromatographic data (equivalent
chain lengths), and mass spectrometric characteristics of all-cis 5-olefinic acids. Eur. J. Lipid Sci. Technol. 104, 234–244. http://dx.doi.org/10.1002/1438-9312(200204)104:4<234::AID-EJLT234>3.0.CO;2-H
|
| ○ |
Wolff RL, Christie WW, Pédrono F, Marpeau AM, Tsevegsüren N, Aitzetmüller K, Gunstone FD. 1999. Δ5-Olefinic Acids in the Seed
Lipids from Four Ephedra Species and Their Distribution Between the α and β Positions of Triacylglycerols. Characteristics Common to Coniferophytes
and Cycadophytes. Lipids 34, 855–864. http://dx.doi.org/10.1007/s11745-999-0433-1
|
| ○ |
Wolff RL, Christie WW, Marpeau AM. 1999. Reinvestigation of the Polymethylene-Interrupted 18:2 and 20:2 Acids of Ginkgo biloba Seed Lipids. J. Am. Oil Chem. Soc. 76, 273–277. http://dx.doi.org/10.1007/s11746-999-0230-0
|
| ○ |
Wolff RL, Pédrono F, Pasquier E, Marpeau AM. 2000. General Characteristics of Pinus spp. Seed Fatty Acid Compositions, and Importance of Δ5-Olefinic Acids in the Taxonomy and Phylogeny of the Genus. Lipids 35, 1−22. http://dx.doi.org/10.1007/s11745-000-0489-y
|
Figure 1. Mass spectrum of the DMOX derivative of unknown FA (X, see Table) from pollen lipids of C. revoluta. Intensities of the remaining fragmentary ions are brought relatively to ion with m/z=113, one of the characteristic ions of FA DMOX derivatives, taken as 100%.