Effects of processing techniques on oxidative stability of Prunus pedunculatus seed oil
J. Yana,b, M.M. Guob,*, Y.H. Shena,*, Y.Y. Wangb, X. Luanb and C. Lia
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry and Materials Science, Northwest University, NO. 229 Taibai North Road, Xi’an 710069, PR China.
bLipids Group, Academy of State Administration of Grain, NO. 11 Baiwanzhuang Street, Xicheng District, Beijing, 100037, PR China.
*Corresponding authors: gmm@chinagrain.org; yhshen@nwu.edu.cn
| |
SUMMARY
This paper investigated the effects of Prunus pedunculatus (P. pedunculatus) seed pre-treatment, including microwaving (M), roasting (R), steaming (S) and roasting plus steaming (RS) on crude oil quality in terms of yield, color change, fatty acid composition, and oxidative stability. The results showed an increase in monounsaturated fatty acid content and oxidative stability of the oils obtained from different processing treatments compared to the oil obtained from raw seeds (RW) without processing. The oils, obtained from pretreated seeds, had higher conjugated diene (CD) and 2-thiobarbituric acid (2-TBA) values, compared to that obtained from RW when stored in a Schaal oven at 65 °C for 168 h. However, polyphenol and tocopherol contents decreased in all oil samples, processed or unprocessed. The effect of pre-treating the seeds was more prominent in the oil sample obtained through the RS technique, and showed higher oxidative stability than the other processed oils and the oil from RW.
|
| |
RESUMEN
Efectos de las técnicas de procesamiento sobre la estabilidad oxidativa del aceite de semilla de Prunus pedunculatus. Se investigó los efectos del pretratamiento de las semillas de Prunus pedunculatus, incluyendo microondas (M), tostado (R), cocción al vapor (S) y torrefacción más vapor (RS), sobre la calidad del aceite crudo, el rendimiento, cambio de color, composición en ácidos grasos y estabilidad oxidativa. Los resultados mostraron un aumento en el contenido de ácidos grasos monoinsaturados y en la estabilidad oxidativa de los aceites obtenidos con diferentes tratamientos de procesamiento en comparación con el aceite obtenido a partir de semillas crudas (RW) sin procesamiento. Los aceites obtenidos a partir de semillas pretratadas presentaron mayores valores de dienos conjugados (CD) y de ácido 2-tiobarbitúrico (2-TBA), comparado con el obtenido de RW cuando se almacenaron en horno a 65 °C durante 168 h. Sin embargo, el contenido de polifenoles y tocoferoles disminuyó en todas las muestras de aceites, procesadas o no procesadas. El efecto del pretratamiento de las semillas fue más destacado en la muestra de aceite obtenida mediante la técnica RS, mostrando mayor estabilidad oxidativa que los otros aceites procesados y que el aceite de RW.
|
1. INTRODUCTIONTOP
Considering the global issue of desertification and its effect on environment, finding effective anti-desertification techniques (Reynolds et al., 2007; Togashi et al., 2013) has become a topic of top priority. Converting desert areas as the base of biological resources by cultivating sand plants could be one of the best methods for desert control. Planting and industrialization can be combined organically by growing economical forests to prevent and control desertification. Some local sand-fixing shrubs, such as Prunus pedunculata Pall., Hippophae rhamnoides Linn., and Pugionium cornutum (L.) Gaertn., have been found to be effective in managing anti-desertification because of their ability to retain local sand, soil, and water (Li et al., 2015). Expanding the scale of planting in arid and semi-arid lands can convert deserts to sustainable land resources. Growing forests by planting sand-fixing shrubs in large scale not only helps to control desertification, but these sand-fixing shrubs are also economically profitable because of their oil content which might inspire farmers to cultivate these shrubs.
Prunus pedunculatus (P. pedunculatus) has been cultivated for centuries, particularly in the north of Shaanxi, the desert of Inner Mongolia. P. pedunculatus has been widely used to tackle de-forestation for preventing desertification in China in recent years (Chu et al., 2013). Moreover, P. pedunculatus seeds contain a high content of edible oil (50.42%) and protein (30.35%), thus making it economically productive. The oil, used as cooking oil, has a mild odor and a pleasant taste; it requires little or no winterization. P. pedunculatus oil contains nearly 97% unsaturated fatty acids and other minor components such as natural antioxidants polyphenol, and tocopherol. P. pedunculatus oil is also rich in monounsaturated fatty acids, predominantly oleic acid; however, it contains much lower amounts of polyunsaturated fatty acids, such as linoleic acid, and small amounts of saturated lipids (Yan et al., 2016). In recent years, more and more consumption of P. pedunculata seeds appear in consumer reports, such as P. pedunculata oil, P. pedunculata nut, and so on, especially because the price of P. pedunculata oil is very expensive on the market.
Oxidative stability is an important parameter for the quality assessment of oil. Atmospheric oxygen affects autoxidation, and the oxidation process proceeds via free radical reactions involving unsaturated fatty acids (Gunstone, 1984). Hydroperoxides, the primary products of autoxidation, subsequently decompose in a series of complex reactions, and the decomposition leads to the formation of a wide range of carbonyl compounds, hydrocarbons, furans and other products that contribute to the stale flavor of foods; it may also be involved in biological oxidation (Frankel, 1987). It has been found that a pleasant aroma and taste, which develop during the roasting process, get transferred to the oil upon extraction (Chandrasekara and Shahidi, 2011). The color of the oil extracted from roasted whole cashew nuts exhibits a higher absorbance value compared to that from raw whole cashew nut (Chandrasekara and Shahidi, 2011). Cisneros et al. (2014) have found that roasting favors the stability of Sacha-inchi oil and they have recommended roasting for extending the shelf life of the oil. Oils, extracted from coated and unhulled seeds after microwaving, are oxidized more quickly than those from other treatments (Abou-Gharbia et al., 1997). Some studies have shown that the chemical composition of oil is independent of the roasting temperature used for its preparation (Kim et al., 2002; Jung et al., 1999).
The objective of our study was to investigate how different processing methods, such as microwaving (M), roasting (R), steaming (S) and roasting plus steaming (RS), of P. pedunculatus seeds before oil extraction affect the properties of the extracted oil. We specifically focused on the following properties: color, fatty acid composition and oxidative stability of the oils during accelerated storage.
2. MATERIALS AND METHODSTOP
2.1. MaterialsTOP
P. pedunculatus seeds were collected in September 2015, from the desert area in Baotou city of the Inner Mongolia Autonomous Region, China. The average length, width, thickness and weight of seed were 11.52 mm, 8.72 mm, 7.65 mm and 0.39 g, respectively. The samples were stored at 4 °C prior to analysis. All chemicals used were of analytical grade. The reference standards for fatty acid, tocopherols (α, β, γ, and δ isomers) were purchased from Sigma-Aldrich.
2.2. Sample preparationTOP
Raw P. pedunculatus seeds (49% water content) were pre-treated employing four different processing methods before extracting oil: microwaving (M): 200 g seeds were put on a tray as a single layer in a microwave oven at 2450 MHz for 10 min; roasting (R) (NATIONAL ENG CO., LTD) at 200 °C for 20 min; steaming (S): 200 g seeds were put on a tray as a single layer, the tray was on the steamer’s perforated strainer, and the seeds were steamed by the bottom boiling water for 20 min; combined roasting (at 100 °C for 15 min) and steaming (at 100 °C for 7 min) (RS). Raw seeds (RW) were used to obtain the unprocessed oil to be used as control.
2.3. Oil extractionTOP
All pre-treated P. pedunculatus seeds (1.5 kg) (samples obtained from four different processing methods) were wrapped inside four layers of filtration cloth and pressed using a laboratory hydraulic press (dimension: 650 mm (L) × 800 mm (D) × 1370 mm (H)) (National Eng. Co., Ltd., Korea). The maximum pressure applied for the mechanical hydraulic press extraction was 60 MPa (for 20 min). The oils collected were centrifuged to remove any particles and then stored at −70 °C prior to analysis. The oil collected from RW (unprocessed) was used as the control.
2.4. Color developmentTOP
The color of all extracted oil samples (RW, M, R, S, and RS) was analyzed spectrophotometrically following the method by Yoshida et al. (1999) with a slight modification. As an index of color development, the absorbance at 420 nm of 5% (w/v) solutions of oils in isooctane was used.
2.5. Oxidative stabilityTOP
Oil samples (25 mL) were placed in 100 mL beakers and stored in the dark in an oven at 65 °C for 168 h. Samples were taken out at regular intervals of 24 h, i.e., after 0 h, 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, and 168 h, and were then stored at 65 °C until used for further analysis.
2.6. Determination of amounts of conjugated dienes and 2-thiobarbituric acidTOP
The contents of conjugated dienes (CD) were determined using the IUPAC 2.505 method (1987). The oil (10–20 mg) was weighed and dissolved in isooctane in a 25 mL volumetric flask. The absorbance at 233 nm was recorded by a UV-visible spectrophotometer (Shimadzu, Japan). Pure isooctane was used as a reference. CD values were calculated based on the following formula: CD = 0.84 × (Absorbance of solution at 233 nm/bc−k0), where c is the concentration of oil in g per 1L, b is the length of the cuvette in cm, and k0 is the absorption of acid (0.03).
The 2-thiobarbituric acid value was determined using the AOCS Cd 19–90 official method (1998). The 2-TBA (200 mg) was dissolved in 1-butanol in a 100 mL volumetric flask and stored in the dark at room temperature for 12 h. Before evaluating the 2-TBA value in the oil samples, an appropriate volume of 2-TBA solution was collected from the stock solution using suction filtration and again dissolved in 1-butanol in a 100 mL volumetric flask. The oil sample (200 mg) was dissolved in 1-butanol in a 25 mL volumetric flask. Next, 5 mL P. pedunculatus oil solution and 5 mL 2-TBA solution were transferred into a screw-capped test tube. The mixed solution was incubated at 95 °C in a thermostated water bath for 2 h, followed by cooling in an ice bath for 10 min. 1-butanol (5 mL) and 2-TBA solution (5 mL) were used as references. The absorbance at 530 nm was recorded using a UV-visible spectrophotometer (Shimadzu, Japan). Results were expressed as mg malondialdehyde (MAD) equivalents per g of oil. 2-TBA values were calculated based on the following formula: 2- TBA = 50 × Absorbance of solution at 530 nm/ the weight of P. pedunculatus oil.
2.7. Fatty acid compositionTOP
Fatty acid composition was determined according to the method described by Bimakr et al. (2013). The oil sample (60 mg) was dissolved in 4 mL isooctane in a test tube and 200 µL 2 mol/L KOH-methanol were added. The mixed solution was then stirred for 30 s and given a standing time of 1–2 min during which 1 g NaHSO4 was added, and again the mixture was stirred for 1 min followed by a standing time of 10 min. The supernatant was finally collected for gas chromatography (GC) analysis. The GC (6890N, Agilent, USA) was equipped with a capillary column (VF-23ms 30 m × 0.25 mm × 0.25 μm, Agilent, USA) and a flame ionization detector (FID). The oven temperature had the following program: 0–40 min at 180 °C followed by 40–60 min at 220 °C, with a rate of 4 °C /min. The FID temperature was maintained at 250 °C. The flow rates of helium, air and hydrogen were 1.0 mL/min, 300 mL/min, and 30 mL/min, respectively.
2.8. Oxidative Stability (OSI value)TOP
OSI values (AOCS Official Method Cd 12b-92) were obtained in duplicate for all oil treatments and the control using the Oxidative Stability Instrument (Omnion, Inc.). The instrument was run using a 3.000 ± 0.0001 g oil sample and a heating block temperature of 110 °C.
2.9. Polyphenol contentTOP
Polyphenol content was determined using the Folin-Ciocalteu assay, following the method by Parry et al. (2005) with a slight modification. The oil sample (2.50 g) was dissolved in 2.5 mL n-hexane in a test tube, and 7.50 mL 80% methanol were added to extract polyphenols. The mixed solution was stirred for 1 min and given a standing time of 9 min, extracted three times at room temperature for 10 min and then centrifuged at 3000 rpm for 10 min to collect the solution; excess methyl alcohol was removed by rotary evaporation at 66 °C. Next, 2.5 mL of 10% Folin-Ciocalteu reagent and 10 mL of 7.5% sodium carbonate solution were added to the solution taken in a 25 mL volumetric flask and made up to volume with distilled water. After 1 h of incubation, the absorbances were measured at 765 nm using a UV-visible spectrophotometer (Shimadzu, Japan). The polyphenol content was expressed in mg of polyphenols per 1000 g of extracted oil.
2.10. Tocopherol contentTOP
Tocopherol content was determined according to AOCS Method (AOCS, 1998) using a HPLC equipped with a fluorescence detector. In brief, 0.25 g oil sample was taken in a 25 mL volumetric flask and diluted in n-heptane. Next, the mixture was filtered with a PTFE filter (pore size 0.45 µm), and 10 µL of the filtrate was injected into the HPLC. The HPLC system was equipped with a LiChroCART @ 250-4 column (250 mm × 4.0 mm), 2695 pump, and 2475 Multi λ Fluorescence Detector (Waters Corporation, USA). Excitation and emission wavelengths were set at 295 nm and 330 nm, respectively. The mobile phase consisted of n-hexane/ tetrahydrofuran (1000/40 by vol.), and a flow rate of 1.0 mL/min was used. The tocopherol content was expressed in milligram of tocopherols per 1000 g of extracted oil.
2.11. Statistical AnalysisTOP
The data are expressed as mean ± standard deviation. The analyses were performed using SPSS (version 17.0 for Windows 2007, SPSS Inc.); results with p ≤ 0.05 were considered as statistically significant
3. RESULTS AND DISCUSSIONTOP
3.1. Oil yieldTOP
We found the crude fat content as high as 52.19 %, accounting for more than half of P. pedunculatus seeds. The oil yields obtained from P. pedunculatus seeds obtained employing different processing treatments ranged from 74.87% to 85.21% (Figure 1). The oils obtained using RS, R, and M methods exhibited significantly higher percentages of oil yield compared with that of unprocessed raw seeds (RW) 72.35%. This might be because of the de-naturation of proteins in P. pedunculatus seeds at higher temperature during process treatments, leading to a decrease in solubility. Due to de-naturation, hydrophobic groups get diverted outwardly, thus exposing and accumulating oil in the globulin on the seeds’ surface; this, in fact, favors oil extraction.
|
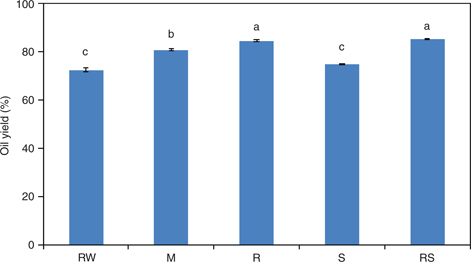 Figure 1. Yields of oil from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)). Figure 1. Yields of oil from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)).
Note: Different letters mean significant differences.
|
|
3.2. Color development of P. pedunculatus oilTOP
The different processing treatments of P. pedunculatus seeds resulted in changes in oil color (Figure 2). The color of P. pedunculatus oil obtained from R seeds did not differ from that of RW seeds at 420 nm; however, the color of the oil obtained from M seeds increased and became significantly darker (p ≤ 0.05) compared with RW, R, S, and RS. This could be due to the products formed in the Maillard reaction during M. treatment (Wu Huiling et al., 2010), or the cell structure of the seeds changed during the M. treatment; the pigment and phospholipids were more likely to be extracted together with the oil (Wang, 2011).
|
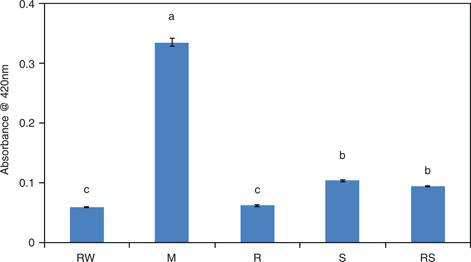 Figure 2. Color of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)). Note: Different letters mean significant differences. Figure 2. Color of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)). Note: Different letters mean significant differences.
Note: Different letters mean significant differences.
|
|
3.3. Fatty acid compositionTOP
Table 1. summarizes the fatty acid composition of the oils extracted from P. pedunculatus unprocessed (RW) and processed (M, R, S, and RS) seeds. As evident from Table 1, the fatty acid compositions of five oil samples (RW, M, R, S, and RS) remained unchanged; however, the difference in content was significant. Oleic acid (C18:1) and linoleic (C18:2) acid were the main fatty acids in all samples, respectively. The main reason for oil rancidity was the oxidation of highly unsaturated fatty acids (USFA); lower content of USFA increases the oil’s stability. Regardless of processing techniques, the USFA content of P. pedunculatus oil was high with the oil sample obtained from RW showing 97.67% USFA. The oil obtained from S seeds contained a high USFA of 98.08%, followed by RS with 97.78%. The oil obtained from RS had a 47.93 ratio of USFA to SFA and the calculated oxidizability (Cox) value of 3.45. The Cox value of the oils was calculated by percentage of unsaturated C18 fatty acids, applying the formula proposed by Fatemi and Hammond (1980):
(Cox=[1×(C18:1%)+10.3×(C18:2%)+21.6×(C18:3%)]/100)
Table 1. Fatty acid composition of oils obtained from P. pedunculatus, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS))
| |
|
RW |
M |
R |
S |
RS |
| SFA |
C16: 0/% |
1.48±0.00b |
1.79±0.00e |
1.63±0.00d |
1.52±0.00c |
1.46±0.00a |
| MUFA |
C16: 1/% |
0.15±0.00a |
0.15±0.00a |
0.15±0.00a |
0.15±0.00a |
0.15±0.00a |
| SFA |
C18: 0/% |
0.59±0.00b |
0.65±0.00d |
0.61±0.00c |
0.59±0.00b |
0.58±0.00a |
| MUFA |
C18: 1/% |
70.32±0.04a |
70.44±0.06a |
70.78±0.01b |
70.91±0.11b |
70.98±0.02b |
| USFA |
C18: 2/% |
26.94±0.01d |
26.61±0.01b |
26.42±0.01a |
26.74±0.04c |
26.37±0.02a |
| USFA |
C18: 3/% |
0.10±0.00a |
0.13±0.00c |
0.13±0.00c |
0.12±0.00b |
0.12±0.00b |
| MUFA |
C20: 1/% |
0.16±0.00a |
0.17±0.00b |
0.17±0.00b |
0.16±0.00a |
0.16±0.00a |
| |
SFA |
2.07 |
2.44 |
2.24 |
2.11 |
2.04 |
| |
MUFA |
70.63 |
70.76 |
71.10 |
71.22 |
71.29 |
| |
USFA |
97.67 |
97.5 |
97.65 |
98.08 |
97.78 |
| |
USFA/SFA |
47.18 |
39.96 |
43.59 |
46.48 |
47.93 |
| |
Cox |
3.50 |
3.47 |
3.46 |
3.49 |
3.45 |
| |
OSI/h |
13.87±0.07a |
17.13±0.04c |
18.78±0.04d |
14.49±0.20b |
19.13±0.18d |
| Results are mean values of three determinations ± standard deviation. Values in each row bearing the same letters are not significantly (P ≥ 0.05) different from one other. |
As the Cox value of oil is lower, its antioxidant ability was higher. In addition, the oil obtained from RS seeds showed the highest stability with an oil stability index (OSI) of 19.13 h. The Oil Stability Index (OSI; AOCS Official Method Cd 12b-92) had largely replaced the AOM as a means to measure an oil’s susceptibility to oxidation (Broadbent et al., 2003). The USFA in P. pedunculatus oil was mainly composed of MUFA (70.91%). Therefore, in the calculation of Cox values, the content of oleic acid was lower than linoleic acid and linolenic acid, suggesting a lower oxidant rate of oleic acid than other C18 in PUFA (Rossi et al., 2007). This indicated better stability of the P. pedunculatus oils extracted from different processing treatments; the Cox values for processed P. pedunculatus oils were less than that of the RW oil, while the OSI value of the oil from RW seeds was the lowest among them all.
3.4. High temperature storage and different processing treatmentsTOP
3.4.1. Conjugated diene valuesTOP
Conjugated dienes (CDs) show the degree of formation of primary and secondary oxidtion products from lipid oxidation due to a shift in double bond positions upon oxidation of methylene interrupted lipid dienes or polyenes (Salunkhe et al., 1991). In the present study, we used CD values to show a well-defined trend in relation to different processing treatments (Figure 3). We found that the CD values of P. pedunculatus oils increased gradually as the storage time increased. The CD values of the oil extracted from M seeds and RW seeds were higher than the CD values for other oils. This may because the two oils were easier to produce hydroperoxides. We found the lowest increase in CD values for the oils obtained from RS and R. Briefly, the CD values for the oil obtained from RW seed were the highest, while those of the oil extracted from RS seed were the lowest upon storage. Thus the oil obtained from RS seeds had better oxidative stability.
|
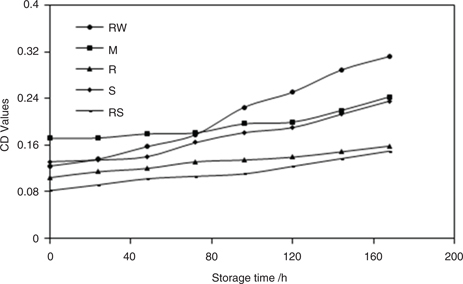 Figure 3. CD values of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), during high-temperature storage at 65 °C. Figure 3. CD values of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), during high-temperature storage at 65 °C.
|
|
3.4.2. 2-thiobarbituric acid (TBA) valuesTOP
The 2-TBA values offer a measurement of the secondary oxidation products in oil. We found that the 2-TBA values (expressed as µg malonaldehyde (MAD) equivalents per g) of oils extracted from RW, M, R, S, and RS seeds increased slowly during the storage period for all samples (Figure 4). The oil from RS seeds showed an insignificant increase in 2-TBA values compared with the other oils from RW, M, R, S seeds during storage. All oils extracted from the different processing treatments had low 2-TBA values. This could be due to the presence of the high γ-tocopherol (551.1–644.88 mg/kg) or the low content of linolenic acid (0.12%–0.23%) in the P. Pedunculatus; meanwhile, TBA were secondary products of lipid oxidation, and require more time to be formed, so that the other reason could be that more TBA haven’t been formed in this storage period. These low 2-TBA levels were in agreement with those previously reported in the literature (Abou-Gharbia et al., 1997; Logani and Davies, 1980).
|
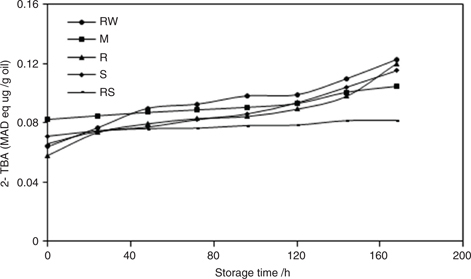 Figure 4. 2-TBA values of oils obtained from P. pedunculatus, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), during high-temperature storage at 65 °C. Figure 4. 2-TBA values of oils obtained from P. pedunculatus, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), during high-temperature storage at 65 °C.
|
|
3.4.3. Polyphenol contentTOP
We found higher polyphenol contents in P. pedunculatus oils obtained from M, R, S, and RS seeds compared with that of the oil obtained from RW seeds (Figure 5). The oil obtained from M seeds had the highest amount of polyphenols (693.47 mg/kg). This indicated that the microwave treatment might have damaged the P. pedunculatus seed cell membrane to allow an increased release of polyphenols (Sowbhagya, 2015.). The polyphenol contents gradually decreased in all the oils with extended storage time. However, we found that polyphenol degradation rates were slower in the oils obtained from processed seeds compared with the oil from RW seeds.
|
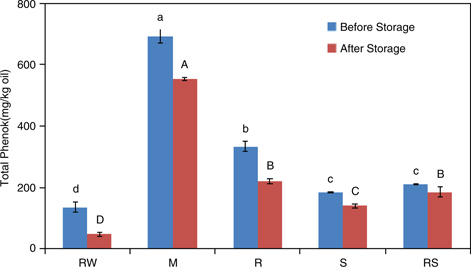 Figure 5. Polyphenol contents of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), before and after high-temperature storage at 65 °C. Figure 5. Polyphenol contents of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), before and after high-temperature storage at 65 °C.
Note: Different small letters mean significant differences, the capitals was the same.
|
|
3.4.4. Tocopherol contentsTOP
Figure 6 shows the decrease in tocopherol contents during the high temperature storage period. We found that the oils obtained from M, R, S, RS seeds had a slightly lower tocopherol content than the oil from RW seeds. This could be due to decomposition of tocopherols in the oils obtained from seeds pre-treated at a high temperature. However, the rate of degradation became lower during storage at a high temperature. This lower degradation rate of tocopherols in P. pedunculatus oils obtained from differently processed seeds seems to result from an additional protection from oxidation due to processing treatments (Cisneros et al., 2014).
|
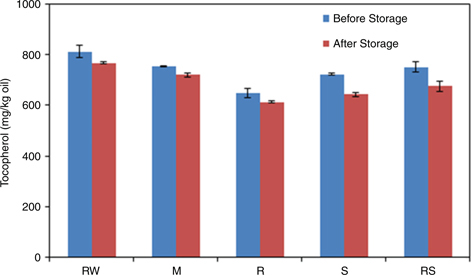 Figure 6. Tocopherol values of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), during high-temperature storage at 65 °C. Figure 6. Tocopherol values of oils obtained from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)), during high-temperature storage at 65 °C.
|
|
4. CONCLUSIONSTOP
In the present investigation on the effects of pre-treatment on P. pedunculatus oil properties, we found that the fatty acid composition remained unchanged regardless of the processing method. The pre-treatment of the seeds increased the unsaturated fatty acid and polyphenol contents of the extracted oils, which in turn seemed to provide some protection to the oil against oxidation during its storage under accelerated oxidation. Although CD and 2-TBA values increased with extending storage time at a high temperature, polyphenol and tocopherol contents decreased. Compared to the oil extracted from RW seeds, the oils extracted from processed seeds exhibited improved oxidation stability with the oil extracted from RS seeds being the most stable. However, the mechanism behind the better stability of the oil obtained from RS seeds needs to be investigated further. In addition, how polyphenol and tocopherol contents positively contribute to the oxidation stability of oil would be an interesting topic to be researched.
ACKNOWLEDGMENTSTOP
This research was funded by Science and Technology Innovation Project Coordination of Shaanxi Province of China (No. 2012KTCL03-05 and 2011KTCL03-04) and the National High-tech Research and Development Projects (863 Torch Program 2013AA102104).
REFERENCESTOP
| ○ |
Abou-Gharbia HA, Shahidi F, Adel A, Shehata Y, Youssef MM. 1997. Effects of processing on oxidative stability of sesame oil extracted from intact and dehulled seeds. J. Am. Oil Chem. Soc. 74, 215–221. http://dx.doi.org/10.1007/s11746-997-0126-9 |
| ○ |
American Oil Chemists’ Society (AOCS). Official Methods and Recommended Practices, 5 th ed.; AOCS Press: Champaign, IL, 1998. |
| ○ |
Bimakr M, Rahman RA, Taip FS, Adzahan NM, Sarker MZI, Ganjloo A. 2013. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules 18, 997–1014. http://dx.doi.org/10.3390/molecules18010997 |
| ○ |
Broadbent CJ, Pike OA. 2003. Oil stability index correlated with sensory determination of oxidative stability in canola oil. J. Am. Oil Chem. Soc. 80, 59–63. http://dx.doi.org/10.1007/s11746-003-0651-y |
| ○ |
Chandrasekara N, Shahidi F. 2011. Oxidative stability of cashew oils from raw and roasted nuts. J. Am. Oil Chem. Soc. 88, 1197–1202. http://dx.doi.org/10.1007/s11746-011-1782-3 |
| ○ |
Chu J, Xu X, Zhang Y. 2013. Production and properties of biodiesel produced from Amygdalus pedunculata pall. Bioresource Technol. 134, 374–376. http://dx.doi.org/10.1016/j.biortech.2012.12.089 |
| ○ |
Cisneros FH, Paredes D, Arana A, Cisneros-Zevallos L. 2014. Chemical composition, oxidative stability and antioxidant capacity of oil extracted from roasted seeds of sacha-inchi (Plukenetia volubilis L.). J. Agric. Food Chem. 62, 5191–5197. http://dx.doi.org/10.1021/jf500936j |
| ○ |
Fatemi SH, Hammond EG. 1980. Analysis of oleate, linoleate, and linolenate hydroperoxides in oxidized ester mixtures. Lipids 15, 379–385. http://dx.doi.org/10.1007/BF02533555 |
| ○ |
Frankel EN. 1987. Secondary products of lipid oxidation. Chem. Phys. Lipids 44, 75–85. http://dx.doi.org/10.1016/0009-3084(87)90045-4 |
| ○ |
Gunstone FD. 1984. Reaction of oxygen and unsaturated fatty acids. J. Am. Oil Chem. Soc. 61, 441–445. http://dx.doi.org/10.1007/BF02678811 |
| ○ |
IUPAC, Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th edn., edited by C. Paquot and A. Hautfenne, International Union of Pure and Applied Chemistry, Blackwell Scientific Publications, United Kingdom 1987, pp. 210–211. |
| ○ |
Jung MY, Bock JY, Baik SO, Lee JH, Lee TK. 1999. Effects of roasting on pyrazine contents and oxidative stability of red pepper seed oil prior to its extraction. J. Agric. Food Chem. 47, 1700–1704. http://dx.doi.org/10.1021/jf981028l |
| ○ |
Kim IH, Kim CJ, You JM, Lee KW, Kim CT, Chung SH, Tae BS. 2002. Effect of roasting temperature and time on the chemical composition of rice germ oil. J. Am. Oil Chem. Soc. 79, 413–418. http://dx.doi.org/10.1007/s11746-002-0498-2 |
| ○ |
Li H, Li C, Zhang C, Chen B, Hui L, Shen Y. 2015. Compositional and gastrointestinal prokinetic studies of Pugionium (L.). Food Chem. 186, 285–291. http://dx.doi.org/10.1016/j.foodchem.2015.03.146 |
| ○ |
Logani MK, Davies RE. 1980. Lipid Oxidation: Biologic Effects and Antioxidants—A Review. Lipids 15, 485–495. http://dx.doi.org/10.1007/BF02534079 |
| ○ |
Parry J, Su L, Luther M, Zhou K, Yurawecz MP, Whittaker P, Yu L. 2005. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 53, 566–573. http://dx.doi.org/10.1021/jf048615t |
| ○ |
Reynolds JF, Smith DMS, Lambin EF, Turner BL, Michael M, Batterbury SPJ. 2007. Global desertification: building a science for dryland development. Science 316, 847–851. http://dx.doi.org/10.1126/science.1131634 |
| ○ |
Rossi M, Alamprese C, Ratti S. 2007. Tocopherols and tocotrienols as free radical-scavengers in refined vegetable oils and their stability during deep-fat frying. Food Chem. 102, 812–817. http://dx.doi.org/10.1016/j.foodchem.2006.06.016 |
| ○ |
Salunkhe DK, Chavan JK, Adsule RN, Kadam SS. 1991. Sesame. In World oilseeds. History, technology and utilization (pp. 371–402). New York: Van Nostrand Reinhold. |
| ○ |
Sowbhagya HB, Lohith DH, Anush SM, Rao LJM. 2015. Microwave Impact on the Flavour Compounds of Cinnamon Bark (Cinnamomum Cassia) Volatile Oil and Polyphenol Extraction. Current Microwave Chem. 2. http://dx.doi.org/10.2174/2213335602666151012193155 |
| ○ |
Togashi S, Obana S, Watanabe S, Horaguchi S, Yashima M, Inubushi K. 2013. Collection screening and evaluation of terrestrial cyanobacterial strains for the bioreclamation of arid soils. Soil Microorganisms 67, 3–9. |
| ○ |
Yan J, Shen Y, Wang Y, Luan X, Guo M, Li C. 2016. The effects of different extraction methods on the physicochemical property and antioxidant activity of Amygdalus pedunculatus seed oil. J. App. Botany Food Quality 89, 135–141. |
| ○ |
Yoshida H, Takagi S, Mitsuhashi S. (1999). Tocopherol distribution and oxidative stability of oils prepared from the hypocotyls of soybeans roasted in microwave oven. J. Am. Oil Chem. Soc. 76, 915–920. http://dx.doi.org/10.1007/s11746-999-0106-3 |
Figure 1. Yields of oil from P. pedunculatus seeds, unprocessed (RW) and processed (microwaving (M) at 2450 MHz, roasting (R) at 200 °C, steaming (S) at 100 °C, roasting at 200 °C plus steaming at 100 °C (RS)).