A Mediterranean-style breakfast increases postprandial serum α-tocopherol levels in lean and obese individuals
S. García-Rodrígueza, L. Sinausiaa, C. Barragána, E. Monterob and J.S. Peronaa,*
aFood and Health, Instituto de la Grasa-CSIC, Seville, Spain.
bEmergency unit, HHUU Virgen del Rocío, Seville, Spain.
*Corresponding author: perona@ig.csic.es
| |
SUMMARY
The aim of this study was to compare the variations in the concentrations of tocopherols and retinol in obese adults in the
postprandial state after the intake of a Mediterranean or Western-style breakfast. The study was designed as a randomized,
controlled intervention trial in the postprandial state, for which 24 male adults (12 obese and 12 of normalweight) were recruited.
After a fat challenge, blood samples were collected at different times postprandially and α-tocopherol, γ-tocopherol and retinol
concentrations were determined in serum by HPLC. The Mediterranean-style meal produced a greater increase in serum α-tocopherol
levels in both obese and normal-weight subjects, compared to the Western-style meal, indicating that the composition of the
food affects the concentration of tocopherols in the postprandial state. However, the serum concentrations of γ-tocopherol
and retinol remained unmodified. In conclusion, the presence of α-tocopherol in the meal could contribute to the protection
of the Mediterranean-style meal against atherosclerosis in the postprandial state.
|
| |
RESUMEN
El desayuno de estilo mediterráneo aumenta los niveles de α-tocoferol sérico posprandial en individuos delgados y obesos. El objetivo de este estudio fue comparar las variaciones en la concentración de tocoferoles y retinol en adultos obesos en
el estado postprandial tras la ingesta de un desayuno mediterráneo u occidental. Consiste en un ensayo de intervención aleatorio
y controlado, en el que participaron 24 adultos varones (12 obesos y 12 normopeso). Tras la ingesta de dichas comidas, se
determinaron las concentraciones de α-tocoferol, γ-tocoferol y retinol en el suero de las muestras sanguíneas mediante HPLC.
La comida de estilo mediterráneo aumentó los niveles séricos de α-tocoferol en sujetos obesos y de peso normal, en comparación
con una comida de estilo occidental, lo que indica que la composición del alimento afecta a su concentración sérica. Sin embargo,
referente al γ-tocoferol y retinol, permanecieron sin modificaciones. En conclusión, la presencia de α-tocoferol en una comida
de estilo mediterráneo podría contribuir a la protección contra la aterosclerosis en el estado postprandial.
|
1. INTRODUCTIONTOP
Obesity is considered one of the main public health concerns in Western countries as it gives rise to other conditions, such
as diabetes or cardiovascular disease. Obesity has also been associated with systemic inflammation and increased oxidative
stress, which lead to decreased α-tocopherol bioavailability, increasing the requirements for this vitamin in obese individuals
(Traber et al., 2017). α-Tocopherol is capable of protecting cell membranes and low density lipoproteins (LDL) from lipid peroxidation (Trpkovic et al., 2015), an alteration that is necessary to trigger atherogenesis (Cabello-Moruno et al., 2007). LDL from obese subjects are more susceptible to oxidation and thus, may require more antioxidants for protection against
oxidative stress (Maruyama et al., 2013). The possible relevance of postprandial α-tocopherol depletion and its relation with lipid peroxidation in diabetic individuals
has been suggested (Manuel-Y-Keenoy et al., 2004). In addition, it has been observed that the intake of antioxidant supplements, such as α-tocopherol, has a greater effect
on oxidative stress in subjects with postprandial anomalies such as hyperglycemia and hyperlipidemia, which are very common
in obese individuals (Plotnick et al., 1997).
There is also considerable evidence that vitamin A homeostasis is involved in regulating body fat and blood glucose levels.
It appears that altered levels of circulating retinol have a relevant role in the modulation of endocrine hormones and gene
transcription associated to lipid and glucose metabolism (Mody 2017). Subjects with a higher adherence to the Mediterranean diet have higher plasma concentrations of β-carotene and α-tocopherol
compared to other dietary patterns, whereas retinol levels remain unchanged (Bach-Faig et al., 2006). However, the effect of a Mediterranean-style breakfast on postprandial serum concentrations of tocopherols and retinol
has not been addressed to date.
This study was designed to determine whether plasma concentrations of retinol and tocopherols (α-tocopherol and γ-tocopherol)
are altered in the postprandial state in obese individuals and whether a Mediterranean-type breakfast can contribute to restoring
such concentrations.
2. MATERIALS AND METHODSTOP
2.1. SubjectsTOP
Twenty-four adult male subjects (22-56 years-old), without a history of digestive or metabolic disorders, were recruited for
the study by placing announcements in online social networks and among acquaintances of the research team in the city of Seville
(Spain). Sample size was calculated by power analysis, considering a type 1 error (α) = 0.05 and a power (1-β) = 0.9 and the
resulting minimum number was 10 individuals per group. Subjects were classified as normal-weight or obese using BMI cut-off
points according to age and sex. Twelve adults were obese (BMI > 30 Kg/m2) and 12 normal-weight (BMI = 20-25 Kg/m2). A written consent form, which was approved by the Institutional Committee on Human Research of HHUU Virgen del Rocío (Seville,
Spain), was obtained from the participants. The consent form and the protocol were in accordance with the institutional and
national ethical standards for human experimentation and the Helsinki declaration of 1975 (revised in 2000). The study was
registered in clinicaltrials.gov (NCT01518803).
2.2. Study designTOP
The study was designed as crossed-over to test the effect of two variables, obesity and meal composition, on α-tocopherol,
γ-tocopherol and retinol serum postprandial concentrations. Participants were asked to have a low-fat dinner the evening prior
to the postprandial assay and to abstain from alcohol drinking and smoking for 24 h. After an overnight fast (12 h), a cubital
vein was catheterized and a baseline blood sample was taken immediately before consumption of the first test meal, which was
consumed in less than 15 minutes. Blood samples were collected at baseline (0h) and at 2h and 4h after the intake of the meals.
During the course of the experiment, the subjects were allowed to drink water and to undertake only light activities. The
subjects consumed a Mediterranean breakfast consisting of 3 slices of brown bread (71 g), 200 mL of skimmed milk, 250 mL of
orange juice and 20 g of mashed tomato and 57 g of olive oil. The composition of the meal was selected to reflect the habitual
breakfast consumption in southern Spain. Olive oil was kindly supplied by Oleicola El Tejar, S.A. (El Tejar, Cordoba, Spain).
The Western breakfast consisted of 55 g of butter, three slices of brown bread and a glass of skimmmed milk (200 ml) with
cocoa powder (20 g). The main composition of the meals is indicated in Table 1. The composition of α-tocopherol and retinol in olive oil and butter was determined as described below for serum and is depicted
in Table 2. The olive oil was directly dissolved in hexane but the butter was first heated at 37 ºC to break the emulsion. The tocopherol
and retinol contents of the other components of the meals were recorded from the Spanish database of food composition (Spanish
Agency for Consumer Affairs, Food Safety and Nutrition).
Table 1. Nutrient compositions of the experimental meals.
| |
Western breakfast |
Mediterranean breakfast |
| Energy (kJ) |
1963 |
2022 |
| Protein (g) |
14 |
15 |
| Carbohydrates (g) |
53 |
62 |
| Sucrose (g) |
26 |
26 |
| Fats (g) |
53 |
57 |
| SFA (g) |
32 |
8 |
| MUFA (g) |
15 |
44 |
| PUFA (g) |
2 |
5 |
| Fiber (g) |
6.4 |
7.0 |
| Cholesterol (mg) |
104 |
0 |
| Tocopherols (mg) |
1.70 |
45.85 |
| SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. |
Table 2. α-Tocopherol and retinol equivalent compositions of the experimental meals.
| |
Western breakfast |
Mediterranean breakfast |
Western breakfast |
Mediterranean breakfast |
| α-Tocopherol (mg) |
Retinol (μg) |
| Brown bread |
0.47 |
0.47 |
ND |
ND |
| Skimmed milk |
0.02 |
0.02 |
ND |
ND |
| Orange juice |
- |
0.43 |
- |
182.5 |
| Tomato |
- |
0.18 |
- |
16.4 |
| Olive oil |
- |
44.75 |
- |
19.4 |
| Cocoa powder |
0.11 |
- |
0.2 |
- |
| Butter |
1.10 |
- |
430.7 |
- |
| Total |
1.70 |
45.85 |
430.9 |
218.3 |
| ND: not detected. - : ingredient not present in the meal. |
2.3. Baseline biochemical analyses TOP
Serum glucose, cholesterol and triglycerides were determined by enzymatic colorimetric methods on a Roche/Hitachi System analyzer
(Roche Diagnostic, Mannheim, Germany). Serum HDL-cholesterol was measured by a direct enzymatic method (HDL-C-plus 2nd generation,
Roche Diagnostics, Mannheim, Germany) on a Roche/Hitachi System analyzer (Roche Diagnostic) and LDL cholesterol was estimated
by the Friedewald equation (Friedewald et al., 1972). Insulin was measured by immunoassay (Abbott Laboratories, Maidenhead, UK) and the Homeostasis Model Assessment of the insulin
resistance (HOMA-IR) score was calculated using the HOMA Calculator software (Diabetes Trial Unit, Churchill Hospital, Oxford,
UK).
2.4. Determination of tocopherols and retinolTOP
Liposoluble vitamins were extracted from 500 μL of serum as follows: 500 μL of ethanol and 1mL of hexane were added sequentially.
The mixture was centrifuged (3500 rpm, 15 ºC for 10 min) and the supernatant was collected. Hexane was evaporated under a
stream of nitrogen and the vitamins were re-dissolved in choloroform/methanol (2:1, v/v) for injection into the the HPLC system.
This system consisted of a reversed-phase column (Novapack) and an elution system composed of methanol/acetonitrile (chloroform
(9:78:13, v/v/v) which run isocratically. A photodiode array detector (PAD 996, Waters) was used for α-tocopherol, γ-tocopherol
and retinol detection at 290 nm (α-tocopherol and γ-tocopherol) and 325 nm (retinol), respectively. These compounds were identified
using commercial standards and quantified by means of external standard.
2.5. Statistical analysisTOP
The results were expressed as mean ± SEM. Data analyses were performed with the GraphPad Prism® 5 statistical package (GraphPad Software Inc., San Diego, CA). The baseline statistical significance of anthropometric measurements,
serum biochemical determinations and serum α-tocopherol, γ-tocopherol and retinol concentrations between obese subjects and
their normalweight counterparts were assessed with the Student’s t test. The same comparisons were made for the incremental
AUC (iAUC) values which were calculated from the postprandial curves. A 2x2 ANCOVA test was applied on iAUC values using the
meals (Mediterranean and Western) and body size as the independent variables. For those comparisons which resulted in significantly
different, a Student’s t test was used to compare the iAUC values. Differences were considered statistically significant at
p < 0.05.
3. RESULTSTOP
3.1. Anthropometric characteristics and serum lipid, glucose, insulin and adipokine concentrationsTOP
Table 3 shows the anthropometric characteristics and fasting serum concentrations of lipids, glucose, insulin and liposoluble vitamins.
Compared to their normal-weight subjects, obese individuals presented higher values of BMI, serum triglycerides, total and
LDL-cholesterol, glucose, and systolic pressure values, but not diastolic blood pressure. In addition, this group presented
lower HDL-cholesterol concentrations. No significant difference was observed for baseline serum concentrations of α-tocopherol
and γ-tocopherol but the retinol content was higher in the obese group.
Table 3. Anthropometric characteristics and baseline serum concentrations of lipids, glucose, insulin and liposoluble vitamins.
| |
Normal-weight |
Obese |
p |
| Age (y) |
27.3 ± 2.9 |
35.8 ± 3.5 |
0.075 |
| Weight (kg) |
74.9 ± 2.3 |
112.9 ± 3.9 |
<0.001 |
| Height (cm) |
179.6 ± 2.0 |
180.8 ± 1.5 |
0.778 |
| BMI (kg/cm2)
|
23.2 ± 0.3 |
34.7 ± 1.4 |
<0.001 |
| Systolic Pressure (mmHg) |
110.9 ± 3.7 |
130.8 ± 2.2 |
<0.001 |
| Diastolic Pressure (mmHg) |
68.9 ± 2.1 |
74.5 ± 2.9 |
0.120 |
| Glucose (mg/dL) |
73.7 ± 1.4 |
83.6 ± 2.4 |
0.002 |
| Insulin (mU/ml) |
4.9 ± 0.8 |
12.6 ± 1.2 |
<0.001 |
| Triglycerides (mg/dl) |
78.7 ± 7.1 |
129.7 ± 17.4 |
0.013 |
| Cholesterol (mg/dl) |
172.5 ± 9.4 |
202.9 ± 10.0 |
0.030 |
| LDL-c (mg/dl) |
94.7 ± 5.6 |
158.7 ± 4.8 |
<0.001 |
| HDL-c (mg/dl) |
57.5 ± 2.8 |
45.9 ± 2.5 |
0.005 |
| α-Tocopherol (μg/ml) |
9.87 ± 0.73 |
10.48 ±1.69 |
0.703 |
| γ-tocopherol (μg/ml) |
0.17 ± 0.03 |
0.14 ± 0.02 |
0.414 |
| Retinol (μg/ml) |
0.45 ± 0.04 |
0.73 ± 0.10 |
0.016 |
| Data are expressed as mean ± SEM; n = 12. BMI: body mass index. LDL-c, low-density-lipoprotein cholesterol; HDL-c, high-density-lipoprotein
cholesterol. Statistical differences were assessed by the Student’s t test. |
3.2. Postprandial concentrations of α-tocopherol and γ-tocopherol and retinol TOP
The postprandial concentrations of the liposoluble vitamins analyzed are depicted in Figure 1. The values were normalized with the concentrations at 0h. For all compounds, except for γ-tocopherol in the obese group,
significant differences were observed 4h after the intake of the meals but not at 2h. The Mediterranean-style breakfast increased
α-tocopherol concentrations at 4h postprandially in both groups (Figures 1A and 1B). In contrast, this breakfast reduced the content of γ-tocopherol (Figure 1C) and retinol (Figure 1E) at that time point compared with the Western-style meal but only in the normalweight group. In obese subjects, no differences
were found for γ-tocopherol between meals (Figure 1D) and the Mediterranean meal increased the content of retinol (Figure 1F).
|
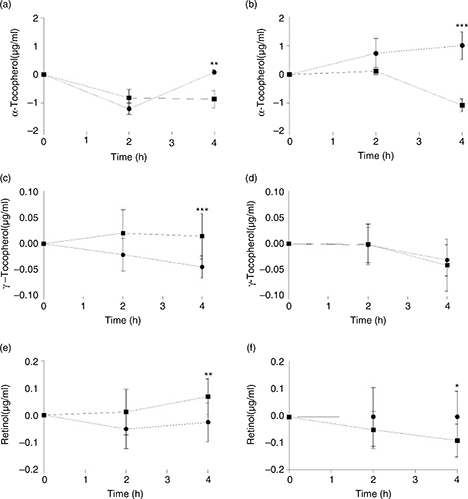 Figure 1. Serum postprandial α-tocopherol, γ-tocopherol and retinol concentrations in the normal weight (1a, 1c and 1e) and obese (1b,
1d and 1f) groups in the postprandial state. Values corresponding to the Mediterranean meal are represented by circle dots,
whereas values corresponding to the Western meal are represented by square dots. Data are shown as mean ± SEM. *: p < 0.05,
**: p < 0.01, ***: p < 0.001. Figure 1. Serum postprandial α-tocopherol, γ-tocopherol and retinol concentrations in the normal weight (1a, 1c and 1e) and obese (1b,
1d and 1f) groups in the postprandial state. Values corresponding to the Mediterranean meal are represented by circle dots,
whereas values corresponding to the Western meal are represented by square dots. Data are shown as mean ± SEM. *: p < 0.05,
**: p < 0.01, ***: p < 0.001.
|
|
As shown in Figure 2, α-tocopherol postprandial iAUC values were significantly higher in both groups after the intake of the Mediterranean meal
(Figure 2A and 2B). In fact, α-tocopherol values were affected by the meal only, and not by body size (Table 4). In contrast, no significant differences were observed in iAUC values for γ-tocopherol and retinol after the intake of the
experimental meals (Figures 2C, 2D, 2E and 2F). These values were not affected by any of the two variables studied (meal and body size) according to 2X2 ANCOVA (Table 4).
Table 4. Impact of body size, type of breakfast and their interaction on the postprandial incremental area under the curve (iAUC) values of the study endpoints.
| |
Body size |
Breakfast |
Body size x breakfast |
| F |
p |
Partial ETA square |
F |
p |
Partial ETA square |
F |
p |
Partial ETA square |
| α-Tocopherol |
0.105 |
0.747 |
0.003 |
7.252 |
0.011 |
0.164 |
0.429 |
0.517 |
0.011 |
| γ-Tocopherol |
0.001 |
0.980 |
0.000 |
0.401 |
0.531 |
0.011 |
0.000 |
0.988 |
0.000 |
| Retinol |
0.059 |
0.809 |
0.001 |
0.173 |
0.680 |
0.004 |
1.396 |
0.244 |
0.034 |
| 2x2 ANCOVA was applied on iAUC calculated from the postprandial profiles for each dependent variable, using body size (normal
weight or obese) and breakfast (Mediterranean or Western) as independent variables. Partial ETA square was used to measure
the effect size of the variable.
|
|
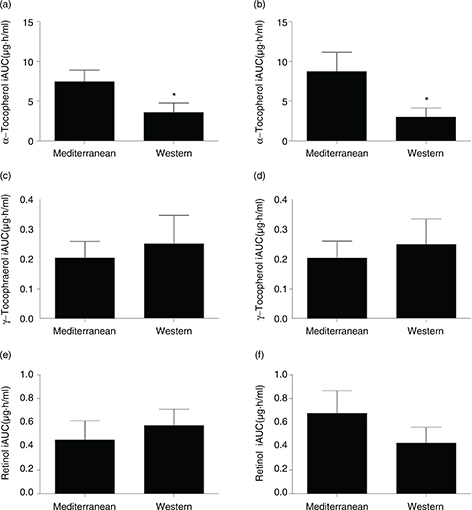 Figure 2. Incremental area under the curve (iAUC) values for serum postprandial α-tocopherol, γ-tocopherol and retinol concentrations
in the normal weight (2a, 2c and 2e) and obese (2b, 2d and 2f) groups. Data are shown as mean ± SEM. *: p < 0.05. Figure 2. Incremental area under the curve (iAUC) values for serum postprandial α-tocopherol, γ-tocopherol and retinol concentrations
in the normal weight (2a, 2c and 2e) and obese (2b, 2d and 2f) groups. Data are shown as mean ± SEM. *: p < 0.05.
|
|
4. DISCUSSIONTOP
We report here that a Mediterranean-style meal can increase postprandial serum α-tocopherol levels in both obese and normal
weight subjects compared to a Western-style meal. The content of α-tocopherol was higher in the Mediterranean breakfast, mainly
due to its content in olive oil (Table 2), but also to orange juice and tomato. Ascorbic acid is also present in orange juice and tomato but its role in protecting
α-tocopherol from oxidation is still controversial. Porkkala-Sarataho et al., (1996) reported that in contrast to α-tocopherol, supplementation with ascorbic acid did not have any significant effect
on VLDL and LDL oxidation, which was confirmed more recently by Van Hoydonck et al., (2004). However, Uzun et al., (2013) demonstrated that vitamin C or its combination with vitamin E significantly increased endothelial vasodilation in patients
with coronary artery disease. Therefore, dietary supplementation with ascorbic acid might not have an impact on α-tocopherol
plasma levels, but they might act synergistically. This can also be the case of lycopene, which is found in relevant concentrations
in tomato. Furhman et al., (2000) found that a combination of lycopene and α-tocopherol showed a greater inhibition of LDL oxidation, compared to
the compounds tested alone, confirming the synergistic effect.
Traber et al., (2015) reported that blood concentrations of α-tocopherol are associated with the mechanisms which control lipid concentrations.
These authors stated that postprandial α-tocopherol remains in circulation for a longer time as the concentration of serum
lipids increases, and that lipoprotein clearance is retarded in obese individuals. Very recently, these authors showed that
subjects with metabolic syndrome displayed higher levels of biomarkers of oxidative stress and inflammation in plasma, suggesting
that α-tocopherol requirements had increased (Traber et al., 2017).
In our study, significant differences in plasma α-tocopherol concentrations between meals were found only at 4 hours postprandially.
At this time point, the size of triglyceride-rich lipoproteins (TRL), which are in fact the transporters of tocopherols and
retinol, is key in atherogenesis (Amigo-Benavent et al., 2016). Cabello-Moruno et al., (2014) observed that triglyceride accumulation was greater when the cells were incubated with the postprandial TRL of intermediate
size which were isolated at 4 h after a lipid load. In this regard, Armengol-Lopez et al., (2012) reported that chylomicron remnants (CMR), the most abundant TRL present in plasma at 4 h postprandially, are pro-inflammatory
and are linked to atherogenic signalling in primary human monocytes. This implies an increased secretion of chemokines and
cytokines, together with the production of the reactive oxygen species (ROS), which can be mitigated by α-tocopherol (Godbout et al., 2004). In addition, α-tocopherol has been shown to reduce the expression of scavenger receptors in macrophages (Teupser et al., 1999), which are essential for CMR uptake (Cabello-Moruno et al., 2014).
Therefore, we believe that postprandial α-tocopherol may play a protective role in serum against atherosclerosis and that
the Mediterranean breakfast favors it positively. In this regard, Carnevale et al., (2014) showed that when extra virgin olive oil was included in a Mediterranean diet, it significantly reduced postprandial oxidative
stress and blocked the decrease in serum levels of vitamin E, which is in agreement with our findings.
Nielsen et al., (2000), studied the effects in the postprandial state of two experimental meals containing different sources of dietary fats (sunflower
oil, rapeseed oil, olive oil, palm oil or butter). They found that the postprandial plasma content of α-tocopherol was significantly
higher after the meal rich in olive and sunflower oils compared to the meal rich in palm oil but the γ-tocopherol content
in VLDL was higher after the rapeseed oil meal. We did not find significant differences in serum γ-tocopherol concentrations
after the consumption of the Mediterranean or Western breakfasts. Bates et al., (2004) reported strong correlations between higher plasma γ-tocopherol levels and obesity indexes, such as body weight, BMI and
waisthip ratio. In addition, Hak et al., (2003) showed that men with high plasma γ-tocopherol levels were more likely to have an increased risk of myocardial infarction.
Therefore, it seems that, in contrast to α-tocopherol, higher plasma γ-tocopherol levels do not exert a protective or favorable
effect against atherosclerosis. However, others have suggested that in healthy individuals, γ-tocopherol supplementation maintains
vascular endothelial function during postprandial hyperglycemia, possibly attenuating lipid peroxidation (Mah et al., 2013).
We also were unable to find differences in serum postprandial retinol concentrations between the groups when the results were
expressed as iAUC. Guerci et al., (2000), found reduced plasma retinyl palmitate at 4 h postprandially in obese individuals after an oral lipid challenge. They argued
that since retinyl palmitate is considered a marker for intestinal TG-rich lipoproteins, the lower concentrations observed
were predominantly due to CMR. Nevertheless, the lack of significant differences found in the present study for retinol is
not surprising because the levels of this biomolecule are highly regulated and vary little with food intake, except in cases
of supplementation or certain pathologies (Ascherio et al., 1992; Hak et al., 2003).
The 2x2 ANCOVA test employed in the present study showed that the type of breakfast affected the levels of α-tocopherol in
plasma but no influence of body size on the postprandial levels of tocopherols and retinol. Although, as stated above, Bates et al., (2004) reported that γ-tocopherol levels were related to obesity, correlations were weak in young individuals like the ones enrolled
in our study. More importantly, they did not have postprandial data. In fact, to our knowledge, our study is the first one
to report postprandial γ-tocopherol levels in obese individuals.
5. CONCLUSIONSTOP
In conclusion, a Mediterranean-style breakfast increases serum α-tocopherol, but not γ-tocopherol or retinol content in both
lean and obese individuals in the postprandial state compared to a Western-style meal. Interestingly, the highest α-tocopherol
concentration was found 4h after the meal intake, a time point at which CMR are most atherogenic. The well-known antioxidant
properties of α-tocopherol together with its reported abilities to down-regulate scavenger receptors in macrophages, could
contribute to the protection of the Mediterranean-style meal against atherosclerosis in the postprandial state.
ACKNOWLEDGMENTSTOP
This work was supported by the Spanish Ministry of Economy, Industry and Competitiveness under Grant AGL2011-23810.
REFERENCESTOP
| ○ |
Amigo-Benavent M, Sinausia L, Montero E, Perona JS. 2016. Discordant ability of the triglyceride to apolipoprotein B ratio
to predict triglyceride-rich lipoprotein particle size in normal-weight and obese men. Exp. Biol. Med. 241, 1772–1775. https://doi.org/10.1177/1535370216639394
|
| ○ |
Armengol Lopez S, Botham KM, Lawson C. 2012. The oxidative state of chylomicron remnants influences their modulation of human
monocyte activation. Int. J. Vasc. Med. 2012. https://doi.org/10.1155/2012/942512
|
| ○ |
Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. 1992. Correlations of vitamin A and E intakes with the
plasma concentrations of carotenoids and tocopherols among American men and women. J. Nutr. 122, 1792–1801.
|
| ○ |
Bach-Faig A, Geleva D, Carrasco J, Ribas-Barba L, Serra-Majem L. 2006. Evaluating associations between Mediterranean diet
adherence indexes and biomarkers of diet and disease. Public. Health Nutr. 9, 1110–1117. https://doi.org/10.1017/S1368980007668499
|
| ○ |
Bates CJ, Mishra GD, Prentice A. 2004. γ-Tocopherol as a possible marker for nutrition-related risk: results from four National
Diet and Nutrition Surveys in Britain. Br. J. Nutr. 92, 137. https://doi.org/10.1079/BJN20041156
|
| ○ |
Cabello-Moruno R, Perona JS, Ruiz-Gutierrez V. 2007. Influence of minor components of olive oils on the composition and size
of TRLs and on macrophage receptors involved in foam cell formation. Biochem. Soc. Trans. 35, 470–471. https://doi.org/10.1042/BST0350470
|
| ○ |
Cabello-Moruno R, Sinausia L, Botham KM, Montero E, Avella M, Perona JS. 2014. Postprandial phase time influences the uptake
of TAG from postprandial TAG-rich lipoproteins by THP-1 macrophages. Br. J. Nutr. 112, 1469–1477. https://doi.org/10.1017/S000711451400244X
|
| ○ |
Carnevale R, Pignatelli P, Nocella C, Loffredo L, Pastori D, Vicario T, Petruccioli A, Bartimoccia S, Violi F. 2014. Extra
virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis. 235, 649–658. https://doi.org/10.1016/j.atherosclerosis.2014.05.954
|
| ○ |
Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma,
Without Use of the Preparative Ultracentrifuge. Clin. Chem. 18, 499–502.
|
| ○ |
Fuhrman B, Volkova N, Rosenblat M, Aviram M. 2000. Lycopene Synergistically Inhibits LDL Oxidation in Combination with Vitamin
E, Glabridin, Rosmarinic Acid, Carnosic Acid, or Garlic. Antioxidants & Redox Signaling 2, 491–506. https://doi.org/10.1089/15230860050192279
|
| ○ |
Godbout J, Berg BM, Kelley KW, Johnson RW. 2004. α-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation
and interleukin-6 secretion in primary murine microglia and in brain. J. Neuroimmunol. 149, 101–109. https://doi.org/10.1016/j.jneuroim.2003.12.017
|
| ○ |
Guerci B, Vergès B, Durlach V, Hadjadj S, Drouin P, Paul JL. 2000. Relationship between altered postprandial lipemia and insulin
resistance in normolipidemic and normoglucose tolerant obese patients. Int. J. Obes. Relat. Metab. Disord. 24, 468–478.
|
| ○ |
Hak AE, Stampfer MJ, Campos H, Sesso HD, Gaziano JM, Willett W, Ma J. 2003. Plasma carotenoids and tocopherols and risk of
myocardial infarction in a low-risk population of US male physicians. Circulation 108, 802–807. https://doi.org/10.1161/01.CIR.0000084546.82738.89
|
| ○ |
Hoydonck PGA Van, Schouten EG, Manuel-y-Keenoy B, Campenhout A Van, Hoppenbrouwers KPM, Temme EHM. 2004. Does vitamin C supplementation
influence the levels of circulating oxidized LDL, sICAM-1, sVCAM-1 and vWF-antigen in healthy male smokers? Eur. J. Clin. Nutr. 58, 1587–1593. https://doi.org/10.1038/sj.ejcn.1601990
|
| ○ |
Mah E, Noh SK, Ballard KD, Park HJ, Volek JS, Bruno RS. 2013. Supplementation of a γ-tocopherol-rich mixture of tocopherols
in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J. Nutr. Biochem. 24, 196–203. https://doi.org/10.1016/j.jnutbio.2012.04.015
|
| ○ |
Majerczyk M, Olszanecka-glinianowicz M, Puzianowska- M, Chudek J. 2016. Retinol-binding protein 4 (RBP4) as the causative
factor and marker of vascular injury related to insulin resistance. Postepy. Hig. Med. Dosw. 70, 1267–1275.
|
| ○ |
Manuel-Y-Keenoy B, Campenhout A Van, Vertommen J, Gaal L Van, De Leeuw I. 2004. Evolution of serum α-tocopherol in the postprandial
and postabsorptive phases in type 1 diabetes mellitus. Ann. N. Y. Acad. Sci. 1031, 439–442. https://doi.org/10.1196/annals.1331.067
|
| ○ |
Maruyama C, Kikuchi N, Masuya Y, Hirota S, Araki R, Maruyama T. 2013. Effects of green-leafy vegetable intake on postprandial
glycemic and lipidemic responses and α-tocopherol concentration in normal weight and obese men. J. Nutr. Sci. Vitaminol. 59, 264–271. https://doi.org/10.3177/jnsv.59.264
|
| ○ |
Mody N. 2017. Alterations in vitamin A/retinoic acid homeostasis in diet-induced obesity and insulin resistance. Proc. Nutr. Soc. 76, 597–602. https://doi.org/10.1017/S0029665117001069
|
| ○ |
Nielsen NS, Marckmann P, Høy C. 2000. Effect of meal fat quality on oxidation resistance of postprandial VLDL and LDL particles
and plasma triacylglycerol level. Br. J. Nutr. 84, 855–863.
|
| ○ |
Plotnick GD, Corretti MC, Vogel RA. 1997. Effect of Antioxidant Vitamins on the Transient Impairment of Endothelium—Dependent
Brachial Artery Vasoactivity Following a Single High-Fat Meal. JAMA J. Am. Med. Assoc. 278, 1682–1686.
|
| ○ |
Spanish Agency for Consumer Affairs, Food Safety and Nutrition A. Spanish database of food composition. http://www.bedca.net/bdpub/index.php. Accessed 11-29-2017.
|
| ○ |
Teupser D, Thiery J, Seidel D. 1999. Alpha-tocopherol down-regulates scavenger receptor activity in macrophages. Atherosclerosis 144, 109–115.
|
| ○ |
Traber MG, Leonard SW, Bobe G, Fu X, Saltzman E, Grusak MA, Booth SL. 2015. α-tocopherol disappearance rates from plasma depend
on lipid concentrations: Studies using deuterium-labeled collard greens in younger and older adults. Am. J. Clin. Nutr. 101, 752–759. https://doi.org/10.3945/ajcn.114.100966
|
| ○ |
Traber MG, Mah E, Leonard SW, Bobe G, Bruno RS. 2017. Metabolic syndrome increases dietary alpha-tocopherol requirements as
assessed using urinary and plasma vitamin E catabolites: a double-blind, crossover clinical trial. Am. J. Clin. Nutr. 105, 571–579. https://doi.org/10.3945/ajcn.116.138495
|
| ○ |
Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. 2015. Oxidized low-density
lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 52, 70–85. https://doi.org/10.3109/10408363.2014.992063
|
| ○ |
Uzun A, Yener U, Cicek OF, Yener O, Yalcinkaya A, Diken A, Ozkan T, Turkvatan A, Ulas M. 2013. Does vitamin C or its combination
with vitamin E improve radial artery endothelium-dependent vasodilatation in patients awaiting coronary artery bypass surgery?
Cardiovasc. J. Afr. 24, 255–259. https://doi.org/10.5830/CVJA-2013-046
|
Figure 1. Serum postprandial α-tocopherol, γ-tocopherol and retinol concentrations in the normal weight (1a, 1c and 1e) and obese (1b,
1d and 1f) groups in the postprandial state. Values corresponding to the Mediterranean meal are represented by circle dots,
whereas values corresponding to the Western meal are represented by square dots. Data are shown as mean ± SEM. *: p < 0.05,
**: p < 0.01, ***: p < 0.001.