Antioxidant and anticancer efficacy of therapeutic bioactive compounds from fermented olive waste
A.E. Mahmouda, S.A. Fathyb, M.M. Alia,*, M.K. Ezzb and A.T. Mohammeda
aBiochemistry Department, Genetic Engineering and Biotechnology Research Division, National Research Centre, Dokki 12622, Giza, Egypt
bBiochemistry Department, Faculty of Science, Ain Shams University, Cairo, Egypt
*Corresponding author: mmali1999@yahoo.com
| |
SUMMARY
Olive pomace, which is considered as one of the worst agro-industrial wastes in Mediterranean countries was tested for bioactive
compounds production through the solid state fermentation of Kluyveromyces marxianus. Because they present potent biological activities, phenolic compounds from both unfermented and fermented pomace were extracted
with simultaneous evaluation of their antioxidant and anticancer activities. Conditions for optimum total phenolic recovery
with maximum antioxidant activity were optimized using methanol as the extracting solvent with a sample to solvent ratio of
1:10 at 50 °C for 2 hours. The in-vitro anticancer activity of both extracts was assessed against different human cancer cell lines. The results revealed that both
extracts exerted anticancer effects close to the value of doxorubicin drug against liver HepG2 and breast MCF-7 cell lines,
and moderate activity against prostate PC3 and colon HCT116 cell lines. Nevertheless, the fermented extract was more potent
than the unfermented one. No effect against lung A549, cervix Hela cancer cell lines or normal HFB4 cells was observed for
both extracts. A GC/MS analysis was carried out to determine the compounds responsible for antioxidant and anticancer activities.
The results showed the presence of methyl palmitate, methyl oleate, and ethyl oleate in the methanolic extract of unfermented
olive pomace, while that of the fermented one showed the production of carvacrol, thymol, eugenol, caryophyllene oxide and
methyl isopalmitate.
|
| |
RESUMEN
Eficacia antioxidante y anticancerígena de compuestos bioactivos terapéuticos de residuos de la fermentación de aceitunas. El orujo de oliva considerado como uno de los peores residuos agroindustriales en los países mediterráneos fue ensayado para
la producción de compuestos bioactivos mediante fermentación en estado sólido de Kluyveromyces marxianus. Se extrajeron los compuestos fenólicos de orujos fermentados y no fermentados ambos con potentes actividades biológicas
y se evaluaron sus actividades antioxidantes y anticancerígenas. Se optimizaron las condiciones para la recuperación fenólica
óptima con actividad antioxidante máxima, estas se lograron usando metanol como disolvente de extracción con una relación
de muestra a disolvente de 1:10 a 50 °C durante 2 horas. La actividad anticancerígena in vitro de ambos extractos se evaluó frente a diferentes líneas celulares de cáncer humano. Los resultados revelaron que ambos extractos
ejercen un efecto anticancerígeno cercano al valor del fármaco doxorrubicina contra líneas celulares hepáticas HepG2 y MCF-7
de mama, y actividad moderada contra líneas celulares de PC3 de próstata y HCT116 de colon, sin embargo, el extracto fermentado
fue más potente que el no fermentado. No se observó ningún efecto contra las líneas celulares A549 de cáncer el pulmón, de
cuello de útero o células HFB4 normales, para ambos extractos. El análisis GC/MS se llevó a cabo para determinar los compuestos
responsables de las actividades antioxidantes y anticancerígenas. Los resultados mostraron la presencia de palmitato de metilo,
oleato de metilo y oleato de etilo en el extracto metanólico de orujo de oliva no fermentado, mientras que el fermentado mostró
la producción de carvacrol, timol, eugenol, óxido de cariofileno e isopalmitato de metilo.
|
1. INTRODUCTIONTOP
Phenolic compounds represent one of the main classes of bioactive compounds which play an important role in cancer prevention
and treatment primarily due to their antioxidant, anticarcinogenic and antimutagenic effects. Dietary polyphenols have received
tremendous attention among nutritionists, food scientists and consumers due to their potent antioxidant properties and their
marked effects in the prevention of various oxidative-stress-associated diseases. Research aiming to find fruits, vegetables,
plants, agricultural and agro-industrial residues as sources of bioactive compounds has been intensified (Dai and Mumper, 2010).
The olive oil industry represents a major seasonal industry in Mediterranean countries with an important economic role. However,
it is associated with the production of a vast amount (2,880,500 tonnes/year) of a solid environmentally polluting residue
known as olive oil cake or olive pomace (OP) (Nunes et al., 2016). The disposal of this waste is a challenging task for olive oil producing countries due to its unfavorable physico-chemical
characteristics (Nunes et al., 2016). Nevertheless, OP is one of the most important agro- industrial sources of phenolic compounds since only 2% of the
polyphenols contained in the olive fruit are transferred into olive oil while the other 98% remain in olive oil by-products
(about 45% of the total phenolics in the olive fruit is retained in OP) (Ciriminna et al., 2016). To date, only a few papers in the literature have focused on the evaluation of the phenolic content of OP as a potential
source of bioactive compounds for pharmaceutical and food industries. Thus, it is of great interest to recover an extract
enriched with phenolic compounds, from the low-cost, environmentally polluting and widely available by-product, OP (Aliakbarian et al., 2011).
Solid State Fermentation (SSF) can be used successfully for the extraction/production of bioactive compounds on industrial
scale for use in food, feed, chemical applications and for the treatment of diseases. In addition, the substitution of synthetic
substrates by agro-industrial residues is considered a valuable approach that does not only eliminate the environmental pollution
caused by these wastes, but also allows for their valorization (Mahmoud et al., 2009; Mahmoud and Ali, 2012).
Scarce research in the literature has focused on the in vitro cytotoxic activity of OP phenolic extracts against different cell lines including HepG2 human hepatoma cell line (Ranchal et al., 2014) and MDA-MB-231 breast cancer cell line (Ramos et al., 2013).
Moreover, a polyphenol extract from OP was proven to restore anoxia-impaired endothelial functions through the regulation
of genes expression more efficiently than the single purified components owing to synergism. Therefore, the combined use of
polyphenols, as in OP extract, could represent a powerful tool for the treatment and chemoprevention of diseases.
This study aimed to recover an extract enriched with phenolic compounds from the environmental polluting agro-industrial waste,
OP, through an eco-friendly technique, SSF, which involves the fermentation of the generally-recognized-as-safe (GRAS) yeast,
Kluyveromyces marxianus NRRL Y-8281. The optimal conditions for phenolic compound extraction from unfermented (UFOP) and fermented OP (FOP) were
studied. The effect of fermentation on the phenolic content and antioxidant activity of OP was assessed. In addition, the
individual components of UFOP and FOP methanolic extracts were identified using the GC/MS technique. Finally, the in vitro anticancer activity of UFOP and FOP methanolic extracts was evaluated against different cell lines.
2. MATERIALS AND METHODSTOP
2. 1. MATERIALSTOP
2.1.1. Olive pomace wasteTOP
The olive pomace (OP) generated from a three-phase decanter system was provided by a local olive-pressing factory located
in Al-Arish, North Sinai, Sinai Peninsula, Egypt, during olive harvesting season. The OP was stored till used in a deep freezer
at −20 °C.
2.1.2. MicroorganismTOP
Kluyveromyces marxianus NRRL Y - 8281 yeast was purchased from Agricultural Research Service (Peoria, Illinois, USA).
2.1.3. Cell lines and culturingTOP
The anticancer impact of the unfermented and fermented extracts was evaluated on HepG2 (liver), MCF-7 (breast), A549 (lung),
Hela (cervix), PC3 (prostate), HCT116 (colon) and HFB4 (normal human melanocyte) cell lines purchased from ATCCT (Rockville,
MD, USA). Maintenance of the cells was achieved as previously described by El Malah et al., (2016).
2.2. METHODS OF ANALYSISTOP
2.2.1. Culture conditionsTOP
Adaptation of yeast was conducted according to Wickerham (1951). The strain was streaked onto a YME/agar medium and then incubated for 48 h at 30 °C. Every 4 weeks the stock was sub-cultured
and stored at 4 °C. The inoculum preparation was done by inoculating a loop of the culture in a sterile inoculum medium (50
ml) of the same composition of the stock medium without agar. The inoculum was then incubated under shaking conditions (150
rpm, for 24 h at 30 °C).
2.2.2. Solid state fermentationTOP
Inoculum of 1 ml (containing about 108 cells/ml) was inoculated into 5 g of sterilized OP (sterilized at 121 °C for 20 min at 15 psi) in Erlenmeyer flasks (250
ml). The Inoculated flasks were incubated statically for 48 h at 45 °C (Mahmoud et al., 2018).
2.2.3. Polyphenol extractionTOP
Both unfermented olive pomace (UFOP) and fermented olive pomace (FOP) were dried in an oven at 50 °C. The dried extracts were
ground by an electrical blender to get powder. The resulting powder was sieved and then stored at −20 °C until use (Mahmoud and Ali, 2012). Phenolic compounds were extracted as reported by Mahmoud and Ali (2012). One gram of dry UFOP or FOP was added to 10 ml of solvent and incubated in a shaking water bath at 100 rpm, 50 °C for 2
h. After extraction, the extracts were filtered and the resulting filtrates were evaporated. The extracted residues were then
re-dissolved in the corresponding solvent to get a concentration of 4 mg/ml. The antioxidant activity and phenolic content
of the reconstituted extracts were evaluated.
2.2.4. Optimization of polyphenol extractionTOP
Various physico-chemical parameters influencing total phenolic recovery were optimized. The influence of solvent type (methanol,
ethanol, distilled water, n-propanol, isopropanol, ethyl acetate, acetone, chloroform and hexane), sample to solvent ratio
(1:5, 1:10, 1:15 and 1:20), extraction time (30 min, 1 h, 2, 4 and 6 h) and extraction temperature (30, 40, 50, 60 and 70
°C) on phenolic content recovery was evaluated. The optimization of various process parameters was to estimate the influence
of each individual parameter (extraction solvent, sample to solvent ratio, extraction time and extraction temperature) regardless
of the others, and subsequently the optimal condition was incorporated into the experiment for optimizing the next parameter.
2.2.5. Total phenolic assayTOP
The extract’s total phenolic content was assessed as mentioned by Quettier-Deleu et al., (2000) as gallic acid equivalent / g dry substrate (mgGAE/gds)
2.2.6. Antioxidant activityTOP
DPPH Radical-scavenging assay. The activity of the extract to scavenge the DPPH radical was evaluated by the method of Mensor et al., (2001).
β-Carotene-linoleic acid assay. Antioxidant activity was evaluated as reported by Juntachote and Berghofer (2005).
2.2.7. Gas chromatographic-mass spectrometric (GC/MS) analysis of UFOP and FOP methanolic extractsTOP
About 5 μl of UFOP and FOP extracts were used. The analysis was performed using Thermo Scientific trace GC Ultra Couple with
single quadrupole MS and a fused silica capillary column TG-5MS (30 m × 0.251 mm, 0.1 mm film thickness). The oven temperature
was maintained initially at 40 °C for 3 minutes and then programmed from 40 to 280 °C with rate of 4 °C/min. Helium was used
as carrier gas, at 1 ml/min flow rate. The determination of all the identified compounds was made using a percent relative
peak area. A tentative identification of the components was made in function with the relative retention time and the mass
spectra with those of The National Institute of Standard and Technology, NIST Willy library data of the GC/MS system.
2.2.8. In vitro anticancer activity assay of UFOP and FOP methanolic extractsTOP
The anticancer activity of UFOP and FOP methanolic extracts (which had maximum total phenolic recovery and the highest antioxidant
activity among other solvents used in the study) was measured as described by Skehan et al., (1990) and Ibrahim et al., (2015) using the Sulfo-Rhodamine-B stain assay. Cells were incubated with serial dilutions of UFOP and FOP extracts or the reference
drug doxorubicin for 48 h. Then the cells were stained with 0.4% acetic acid SRB solution. Unbound stain was washed with acetic
acid. Optical density was measured in an ELISA reader at wave length 545 nm and the inhibitory concentration (IC50) was assayed in comparison with doxorubicin (Ali et al., 2014).
2.3. Statistical analysisTOP
The data were reported as mean ± standard error and analyzed statistically using independent sample t-test for comparison between the fermented and unfermented olive pomace groups. Significance was calculated at p < 0.05. The
correlation coefficient (r) was also calculated by the Bivariate Correlation Test using the SPSS 16.0 Program.
3. RESULTS AND DISCUSSIONTOP
Phenolic compounds represent a major class of bioactive compounds. They are considered as potent antioxidants which can improve
human health through the prevention of heart diseases, inflammation reduction and lowering the incidence of several diseases
such as cancers and diabetes (Khoddami et al., 2013). Research has been intensified aiming to find natural, cheap and renewable sources of bioactive compounds. Therefore, a
comparative study between UFOP and OP fermented by K. marxianus NRRL Y-8281 was conducted in order to investigate the effect of fermentation on OP total phenolic content and antioxidant
activity.
The solvent type which ensures optimum phenolic recovery depends mainly on the chemical properties of the phenolic components
contained in the sample at hand (Khoddami et al., 2013). The chemical nature of phenolic compounds present in the sample determines their solubility, which is governed by the number
of hydroxyl groups and molecular weight. The solubility of total phenolic compounds in turn determines the polarity of solvent
used for total phenolic extraction. Only the mixture of phenolic compounds which is soluble in the solvent selected can be
extracted from the sample. Thus, different solvents extract different proportions of total phenolic compounds from the sample
giving different total phenolic yields and antioxidant activities. The highest total phenolic recovery is usually achieved
using ethanol, methanol and their mixtures with water (Dai and Mumper, 2010). The total phenolic recovery from UFOP and FOP varied in response to different solvents as shown in Figure 1. Solvent type was found to be significantly correlated to phenolic recovery from both UFOP (r =0.552) and FOP (r =0.462). Polar solvents were more efficient in polyphenolic extraction and gave higher yields. Methanol showed the highest
total phenolic recovery (207.35 and 114.78 mgGAE/gds for UFOP and FOP, respectively). Other solvents showed lower total phenolic yield and ranged from 30.66 to 90.25 mgGAE/gds for both UFOP and FOP. These result resemble those reported by Lafka et al., (2011) to some extent. Different extracts showed different antioxidant activity (Figure 2). This is because the antioxidant activity is influenced by the type and polarity of the extracting solvent as well as the
extraction procedure (Lafka et al., 2011). Also, DPPH radical scavenging activity is influenced by both the chemical structure of the scavenger compound and the polarity
of the reaction medium (Uribe et al., 2014). A significant correlation was found between solvent type and the antioxidant activity of UFOP (r =0.892) extract and FOP extract (r =0.898). Methanolic and ethanolic extracts showed the highest antioxidant activity (methanolic extracts expressed 96 and
97% and ethanolic extracts expressed 83.87 and 92.1% for UFOP and FOP, respectively); while the antioxidant activity of ethyl
acetate extract (44.05 and 44.92% for UFOP and FOP, respectively) was surpassed by that of n-propanol (58.28 and 69.96% for
UFOP and FOP, respectively) and iso-propanol (50.53 and 59.39% for UFOP and FOP, respectively). These findings are in accordance
with those reported by Lafka et al., (2011). To explore the influence of total phenolic content on antioxidant activity of OP, the correlation between the antioxidant
capacity and total phenolic content was determined. The antioxidant activity of both extracts was significantly correlated
with their phenolic content (r = 0.807 for UFOP, and r = 0.708 for FOP), which means that phenols represent a high percentage of the antioxidant capacity of olive pomace extract.
A correlation between the antioxidant activity and total phenolic content of OP methanolic extract (r = 0.453) was previously reported by Uribe et al., (2014) and Alu’datt et al., (2010) (r = 0.746). Increasing sample/solvent ratio increased total phenolic recovery. However, the use of large amounts of solvents
is not economical and is environmentally polluting due to the increased solvent waste. Moreover, the appropriate amount of
extracting solvent should be determined to avoid saturation effects (Dai and Mumper, 2010). The data in Figure 3 shows that a sample/solvent ratio of 1:10 was optimum for both total phenolic recovery (207.35 and 114.78 mgGAE/gds for UFOP and FOP, respectively) and antioxidant activity (96 and 97% for UFOP and FOP, respectively). The lowest total
phenolic yield (74.72 and 71.44 mgGAE/gds for UFOP and FOP, respectively) and antioxidant activity (95.69 and 96.4% for UFOP and FOP, respectively) were obtained
when a sample/solvent ratio of 1:5 was applied. This result coincides with the results of Lafka et al., (2011). No significant correlation was found between sample to solvent ratio and total phenolic recovery or antioxidant activity
of the extracts. However, the antioxidant activity of the extracts was strongly and significantly correlated to their phenolic
contents (r = 0.777 for UFOP and r = 0.759 for FOP).
|
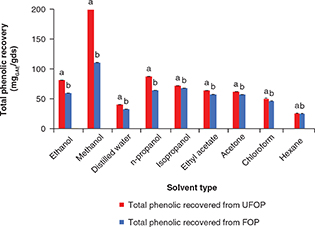 Figure 1. Effect of solvent type on total phenolic recovery from unfermented (UFOP) and fermented (FOP) olive pomace. Figure 1. Effect of solvent type on total phenolic recovery from unfermented (UFOP) and fermented (FOP) olive pomace.
The experiment
was carried out in triplicate. Values are given as mean±standard error of three batches. Independent sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
|
|
|
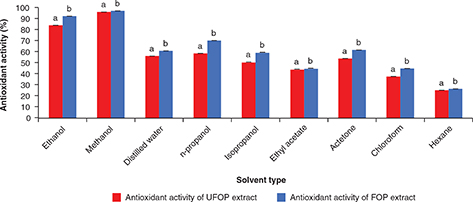 Figure 2. DPPH free radical scavenging activity of unfermented (UFOP) and fermented (FOP) olive pomace extracts obtained with different
solvents. Figure 2. DPPH free radical scavenging activity of unfermented (UFOP) and fermented (FOP) olive pomace extracts obtained with different
solvents.
The experiment was carried out in triplicate. Values are given as mean ± standard error of three batches.
Independent
sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
|
|
|
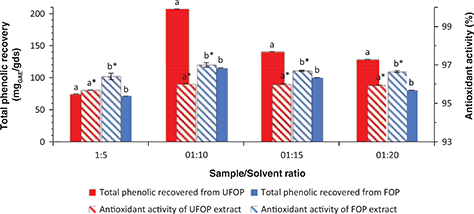 Figure 3. Effect of sample to solvent ratio on total phenolic recovery and antioxidant activity from unfermented (UFOP) and fermented
(FOP) olive pomace. Figure 3. Effect of sample to solvent ratio on total phenolic recovery and antioxidant activity from unfermented (UFOP) and fermented
(FOP) olive pomace.
The experiment was carried out in triplicate. Values are given as mean ± standard error of three batches.
Independent
sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
a and b are significance of means of total phenolic recovered, while a* and b* are significance of means of antioxidant activity.
|
|
The time of total phenolic extraction is a key factor as it influences both the efficiency of the process through maximizing
the extraction yield and the process cost (Aliakbarian et al., 2011). Both extraction time and temperature are responsible for total phenolic solubilization in the extracting solvent and subsequent
extraction. However, both factors are also responsible for analyte oxidation and degradation leading to decreased total phenolic
recovery. Increasing extraction time and temperature normally leads to increased phenolic recovery, as a result of enhanced
analyte solubility, to a certain limit, above which the phenolic yield decreases due to phenolic degradation or oxidation
as a result of exhausted extraction times and high temperatures (Khoddami et al., 2013). Total phenolic recovery as well as antioxidant activity were directly proportional to the extraction time till optimum
recoveries (207.35 and 114.78 mgGAE/gds for UFOP and FOP, respectively) and antioxidant activity (96 and 97% for UFOP and FOP, respectively) were reached at
2 h of extraction, after which they were inversely proportional to further increase in extraction time as shown in Figure 4. Similar behavior was reported by Aliakbarian et al., (2011), who stated a shorter extraction time (90 minutes) due to the application of high pressure and high temperature. While, Lafka et al., (2011) observed an increase in total phenolic recovery with time up to 3 h of extraction. Alu’datt et al., (2010) reported an optimum extraction time of 12 h using methanol. The decrease in antioxidant activity of samples with increasing
the extraction time beyond the optimum one may be due to a loss in the antioxidant activity of polyphenolic compounds by exposure
to temperature, light and oxygen. The same pattern was reported by Lafka et al., (2011). Extraction time was significantly correlated with total phenolic extraction from FOP (r = -0.626), while it was not correlated with total phenolic extraction from UFOP. However, the time of extraction was significantly
correlated with the antioxidant activity of both extracts (r = 0.685 for UFOP and r = 0.624 for FOP). Meanwhile, the antioxidant activity of both extracts was not correlated with their phenolic contents.
|
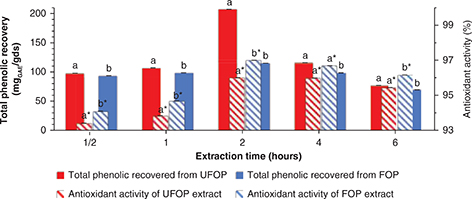 Figure 4. Effect of extraction time on total phenolic recovery and antioxidant activity from unfermented (UFOP) and fermented (FOP)
olive pomace. Figure 4. Effect of extraction time on total phenolic recovery and antioxidant activity from unfermented (UFOP) and fermented (FOP)
olive pomace.
The experiment was carried out in triplicate. Values are given as mean ± standard error of three batches.
Independent
sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
a and b are significance of means of total phenolic recovered, while a* and b* are significance of means of antioxidant activity.
|
|
High extraction temperature usually minimizes the extraction duration. However, elevated temperature can cause phenolic degradation
(Dai and Mumper, 2010). Increasing the extraction temperature not only increases total phenolic solubility and mass transfer rate (diffusion coefficient),
but also decreases the viscosity and surface tension of the solvent used helping the solvent to penetrate the sample particles
leading to an improved total phenolic recovery. Also, high temperature disrupts the strong solute-matrix interactions stabilized
by Vander-Waals forces, hydrogen bonding and dipole attractions, thus allowing for better recovery (Aliakbarian et al., 2011). However, many phenolic compounds could be lost during their extraction at high temperature for prolonged time due to their
oxidation and hydrolysis leading to a decreased recovery (Dai and Mumper, 2010). The data in Figure 5 shows that total phenolic recovery as well as antioxidant activity increased by increasing extraction temperature up to 50
°C (207.35 and 114.78 mgGAE/gds expressing 96% and 97% antioxidant activity for UFOP and FOP, respectively) and then started to fall. This positive relationship
between total phenolic recovery from OP and temperature has been confirmed by other authors. Alu’datt et al., (2010) reported a continuous increase in total phenolic recovery and antioxidant activity with temperature giving maximum recovery
at 70 °C and maximum antioxidant activity at 60 °C. An optimum temperature of 180 °C, giving a maximum total phenolic recovery
using a high pressure-high temperature reactor was recorded by Aliakbarian et al., (2011). On the other hand, Shahidi and Naczk (2003) observed that the extraction temperature was directly proportional to the total phenolic recovery, while it was inversely
proportional to the antioxidant activity. Extraction temperature was strongly correlated with phenolic recovery (r = -0.766 for UFOP and r = -0.813 for FOP) but not correlated with the antioxidant activity of the extracts. However, the total phenolic content of
both extracts was significantly correlated with their antioxidant activity (r = 0.601 for UFOP and r = 0.744 for FOP). Alu’datt et al., (2010) affirmed that the total phenolic compound content of olive pomace extracts varied significantly (p < 0.05) for the tested
temperature range.
|
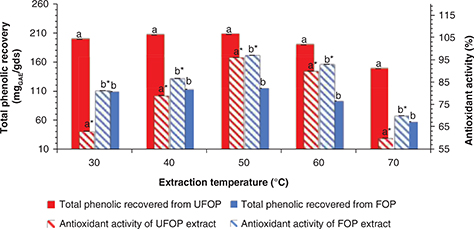 Figure 5. Effect of extraction temperature on total phenolic recovery and antioxidant activity from unfermented (UFOP) and fermented
(FOP) olive pomace. Figure 5. Effect of extraction temperature on total phenolic recovery and antioxidant activity from unfermented (UFOP) and fermented
(FOP) olive pomace.
The experiment was carried out in triplicate. Values are given as mean ± standard error of three batches.
Independent sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
a and b are significance of means of total phenolic recovered, while a* and b* are significance of means of antioxidant activity.
|
|
Further studies were conducted for the determination of the best UFOP and FOP methanolic extract concentration which gives
the optimum phenolic content to express maximum antioxidant activity. The total phenolic content of the methanolic extract
of UFOP and FOP with various concentrations (2–10 mg/ml) is shown in Figure 6. The results showed that the higher the concentration used for the assay, the higher the total phenolic content. However,
the total phenolic content of the FOP extract at all studied concentrations was lower than that of the UFOP extract indicating
OP polyphenol degradation by K. marxianus fermentation. The antioxidant activities of different extract concentrations (2–10 mg/ml) were measured using DPPH radical
scavenging activity and β -carotene/linoleic acid system assays. The results gathered in Figure 7 show that the free radical scavenging activities of both extracts were concentration-dependent. There was a gradual increase
in the DPPH radical scavenging activity of both extracts with an increase in the extract concentration till maximum radical
scavenging activities (96.0 and 97.0% for UFOP and FOP, respectively) were reached at extract concentrations of 4 mg/ml for
both extracts. Beyond this concentration a decrease in their activities was observed. Figure 8 shows that the inhibition of lipid peroxidation activity of both extracts was also concentration-dependent. The inhibition
of lipid peroxidation activity in both extracts increased gradually with an increase in the extract concentration till maximum
inhibition of lipid peroxidation activity (44.5 and 68.9% for UFOP and FOP, respectively) was reached at the extract concentration
of 4 mg/ml for both extracts and above this concentration a decrease in their activities was observed. The decrease in the
antioxidant activity in both extracts observed for both the DPPH radical scavenging system and β - carotene/linoleic acid
system can be attributed to the pro-oxidant activity of phenolic compounds. The concentration of the phenolic-rich extract
was significantly correlated with the total phenolic content (r = 0.992 and 0.991 for UFOP and FOP, respectively) and to the antioxidant activity in the β -carotene/linoleic acid system
assay (r = −0.707 and −0.526 for UFOP and FOP, respectively), although it was not correlated with the DPPH radical scavenging activity
of both extracts. The phenolic content of the UFOP extract was significantly different from that of the FOP extract at sample
concentrations ranging from 4 to 10 mg/ml (p < 0.05), which indicates phenolic degradation of OP by fermentation. No significant difference was found between their
phenolic contents at a concentration of 2 mg/ml. Also, a significant difference (p < 0.05) was found between the DPPH radical scavenging activity of UFOP and FOP methanolic
extracts at different concentrations, indicating an enhanced radical antioxidant activity of OP after fermentation. In addition,
a significant difference (p < 0.05) was found between both extracts with regards to their activity in the β-carotene/linoleic acid system, suggesting enhanced pomace protecting activity against lipid peroxidation after fermentation.
|
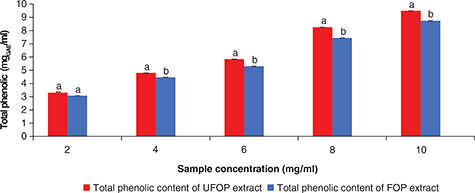 Figure 6. Total phenolic content of unfermented (UFOP) and fermented (FOP) olive pomace methanolic extracts at different concentrations. Figure 6. Total phenolic content of unfermented (UFOP) and fermented (FOP) olive pomace methanolic extracts at different concentrations.
The experiment was carried out in triplicate. Values are given as mean ± standard error of three batches.
Independent sample
t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
|
|
|
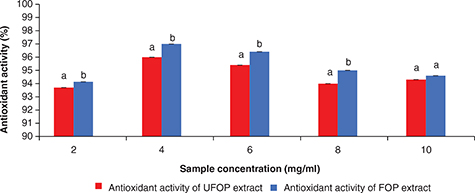 Figure 7. DPPH free radical scavenging activity of different concentrations of unfermented (UFOP) and fermented (FOP) olive pomace methanolic
extracts. Figure 7. DPPH free radical scavenging activity of different concentrations of unfermented (UFOP) and fermented (FOP) olive pomace methanolic
extracts.
The experiment was carried out in triplicate. Values are given as mean ± standard error of three batches. Independent
sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
|
|
|
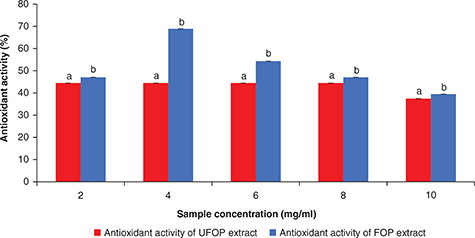 Figure 8. Antioxidant activity of different concentrations of unfermented (UFOP) and fermented (FOP) olive pomace methanolic extracts
using β-carotene / linoleic acid system assay Figure 8. Antioxidant activity of different concentrations of unfermented (UFOP) and fermented (FOP) olive pomace methanolic extracts
using β-carotene / linoleic acid system assay
The experiment was carried out in triplicate. Values are given as mean ± standard
error of three batches.
Independent sample t-test was used for comparison of means. Means bearing different superscripts are significantly different (p < 0.05).
|
|
The optimization of extraction parameters resulted in a maximum total phenolic yield of 207.35 and 114.78 mgGAE/gds for UFOP and FOP, respectively, using methanol with a sample/solvent ratio of 1:10 (V/W) at 50 °C for 2 h at a sample
concentration of 4 mg/ml. Aliakbarian et al., (2011) obtained a maximum total phenolic yield of 45.2 mgGAE/gds using methanol with a ratio of 10:1 (V/W) at 180 °C for 90 minutes under an appropriate pressure. While Alu’datt et al. (2010) reported a maximum total phenolic yield (4.4 mgGAE/gds) using methanol (25:1 V/W) at 70 °C for 12 h. Suárez et al., (2009) reported a maximum total phenolic yield of 2.5 mg phenol/g using ethanol/water (80:20) at 80 °C. From the results obtained
it can be concluded that fermentation of OP for 48 h using K. marxianus NRRL Y- 8281 led to a decrease in total phenolic content from 207.35 mgGAE/gds to 114.78 mgGAE/gds for UFOP and FOP, respectively (representing 44.64% reduction in total phenolic content), along with a small increase
in DPPH radical scavenging activity from 96.0 to 97.0% for UFOP and FOP, respectively. The reduction in total phenolic content
of OP by fermentation was also confirmed by Fadel and El-Ghonemy (2015) who stated a reduction in OP total phenolic content from 3.1% to 0.92% using A. oryzae FK-923 for 7 days.
The reduction in total phenolic quantity while maintaining a nearly constant or slightly elevated DPPH radical scavenging
activity can be attributed to the altered quality of phenolic compounds contained in the FOP. This finding confirms that total
phenolic content does not necessarily increase simultaneously with DPPH scavenging activity as the later depends mainly on
the quality rather than quantity of the phenolic compounds present in the extract. In their study, Uribe et al., (2014) found that the total phenolic content was inversely proportional to the antioxidant activity, suggesting that a detailed
analysis of the quality of polyphenolic compounds present in the sample is a must to identify the compounds responsible for
the high antioxidant activity in samples. In order to present a more precise explanation for total phenolic content-independent
increase in the antioxidant activity of FOP, further investigation and identification of individual compounds present in both
UFOP and FOP was conducted by GC/MS analysis. The data obtained from the GC/MS analysis (Table 1) revealed that the major compounds that could be found in the methanolic extract of UFOP were identified as oleic acid methyl
ester (53.04%), oleic acid ethyl ester (14.17%) and methyl palmitate (11.03%).
Table 1. Compounds identified from the major retention peaks obtained by GC/MS analysis of unfermented olive pomace (UFOP)
methanolic extract
| Compound |
Synonyms |
Retention time (minutes) |
Molecular weight |
Area (%) |
| Hexadecanoic acid, methyl ester |
Palmitic acid, methyl ester
Methyl palmitate |
25.11 |
270 |
11.03 |
| 9-Octadecenoic acid, methyl ester |
Oleic acid, methyl ester
Methyl oleate |
27.13 |
296 |
53.04 |
| 9-Octadecenoic acid, ethyl ester |
Oleic acid, ethyl ester
Ethyl oleate |
27.87 |
310 |
14.17 |
The dominant compound found in UFOP methanolic extract, methyl oleate, was reported to have antioxidant and anticancer activities
(Akpuaka et al., 2013). It has also been reported to serve as an endogenous ligand to peroxisome proliferator-activated receptors. The peroxisome
proliferator- activated receptors are a group of nuclear receptor proteins that function as transcription factors regulating
the expression of genes involved in the regulation of carbohydrate, lipid and protein metabolism, energy homeostasis, cellular
differentiation, tumorigenesis, lung diseases, obesity, diabetes, neurodegenerative disorders, fertility and reproduction
(Tyagi et al., 2011). The second dominant compound found in UFOP methanolic extract, ethyl oleate, is a fatty acid ester formed by the condensation
of oleic acid and ethanol. It is considered an important compound in the food industry as a food additive in addition to its
use for drying fruit. In the pharmaceutical industry, ethyl oleate is used as a solvent for pharmaceutical drug preparations
involving lipophilic substances such as steroids (Aly et al., 2016). Methyl palmitate is considered an inhibitor to 5-Alpha reductase, an enzyme whose inhibitors can be used in benign prostatic
hyperplasia and prostate cancer (Hema et al., 2011; Akpuaka et al., 2013). It is also considered as a hypocholesterolemic factor and antioxidant (Hema et al., 2011).
The results obtained from the GC/MS analysis of UFOP methanolic extract are in line with those obtained by other researchers.
Ethyl oleate and methyl oleate were detected in OP by Kuley et al., (2017). In addition, Schievano et al., (2015) reported the presence of methyl palmitate in OP. On the other hand, carvacrol (4.9%), thymol (2.97%), eugenol (2.87%) and
caryophyllene oxide (1.36%) were detected only in the methanolic extract of FOP (Table 2). However, oleic acid methyl ester (47.42%) and oleic acid ethyl ester (11.93%) were the major components in the extract
followed by methyl- isopalmitate (9.75%). Carvacrol and thymol are monoterpenoid phenolic compounds produced naturally by
some plants (Özkan and Erdoğan, 2011). Several in vitro and in vivo studies suggest that carvacrol has anti-inflammatory, immunomodulatory, antioxidant and anticancer activities. It also possesses
anti- obesity, hepatoprotective, gastroprotective, neuroprotective (anti-Alzheimer disease) and platelet antiaggregating activities.
Clinically, thymol is used as antipyretic, antispasmodic, antihyperlipidemic, antihyperglycemic, gastroprotective, hepatoprotective,
antioxidant, analgesic, anesthetic, antiepileptic and anti-inflammatory (Özkan and Erdoǧan, 2011). Eugenol is an organic phenolic phytochemical. Regarding its minimal toxicity and low side effects, eugenol is widely applied
in the pharmaceutical industry, food industry (as flavoring agent), in cosmetics and in dentistry in addition to being an
antiparasitic and insect repellent (Kong et al., 2014). Caryophyllene oxide is an oxygenated sesquiterpene that expresses numerous pharmacological effects including antimicrobial,
immunomodulatory, antirheumatic, anti-arthritic and antipyretic activities. It is also considered as anti-inflammatory, anti-platelet
aggregation, antioxidant and anticancer agent (Park et al., 2011). Thymol, carvacrol, eugenol and caryophyllene oxide are classified as aromatic compounds which are volatile compounds and
have odor or taste. The detection of these aromatic constituents in the methanolic extract of FOP can be attributed to their
synthesis and excretion by K. marxianus. This strain has been reported to produce aromatic compounds such as monoterpenes, fruit esters, ketones, carboxylic acids,
furans and alcohols (Wilkowska et al., 2015). Although several yeast strains have been reported for the production of aromatic substances, only those stated as GRAS,
such as K. marxianus, can find industrial applications (Fonseca et al., 2008). These findings open new prospects for the possibility of utilizing K. marxianus fermented OP in food, feed and pharmaceutical industries.
Table 2. Compounds identified from the major retention peaks obtained by GC/MS analysis of fermented olive pomace (FOP) methanolic extract
| Compound |
Synonyms |
Retention time (minutes) |
Molecular weight |
Area (%) |
| Phenol,2-methyl-5-(1-methyl-ethyl) |
Carvacrol |
16.58 |
150 |
4.90 |
| Phenol,5-methyl-2-(1-methyl-ethyl) |
Thymol |
16.70 |
150 |
2.97 |
| Phenol,2-methoxy-4-(2-propenyl) |
Eugenol |
17.94 |
164 |
2.87 |
| Caryophyllene -oxide |
- |
20.65 |
220 |
1.36 |
| Pentadecanoic acid, 14-methyl-, methyl ester |
Methyl-isopalmitate |
25.08 |
270 |
9.75 |
| 9-Octadecenoic acid, methyl ester |
Oleic acid, methyl ester
Methyl oleate |
27.13 |
296 |
47.42 |
| 9-Octadecenoic acid, ethyl ester |
Oleic acid, ethyl ester
Ethyl oleate |
27.88 |
310 |
11.93 |
As shown in Figure 9, the antiproliferative activity of UFOP and FOP methanolic extracts was tested using the SRB assay in MCF-7, HepG2, A549,
Hela, PC3 and HCT116 cancer cell lines as well as the normal human melanocyte, HFB4. For comparison, treatment with doxorubicin
was used as a positive control and treatment with DMSO was used as a negative control. The tumor cells showed normal growth
in the culture system. In addition, UFOP and FOP exhibited no activity against the growth of the normal HFB4 cell line. From
the results, it is evident that although UFOP and FOP methanolic extracts displayed potent anticancer activity against HepG2
and MCF-7, they had moderate activity against PC3 and HCT116 cell lines. In addition, they did not exert any activity against
lung A549 or Hela cancer cell lines. In the case of HepG2, UFOP and FOP exerted antiproliferative activity with IC50 values of 22.65 ± 2.48 and 20.00 ± 2.32 μg/ml near the IC50 of the reference drug, doxorubicin (IC50: 20.30 ± 2.34 μg/ml). For MCF-7, the IC50 was 25.50 ± 2.67 and 23.25 ± 2.43 μg/ml (doxorubicin IC50: 24.00 ± 2.72 μg/ml). For PC3 the IC50 was 38.00 ± 5.06 and 32.90 ± 4.62 μg/ml for UFOP and FOP, respectively (doxorubicin IC50: 18.47 ± 2.00 μg/ml). For HCT116 the IC50 was 33.88 ± 3.96 and 32.40 ± 4.13 μg/ml for UFOP and FOP, respectively (doxorubicin IC50: 19.83 ± 2.11 μg/ml). In conclusion, it is clear that, while the IC50 of both UFOP and FOP was close to the value of the doxorubicin in the two cell lines (HepG2 and MCF-7), FOP was more potent
than UFOP in the four cell lines (MCF-7, HepG2, PC3 and HCT116). This enhanced anticancer activity after fermentation can
be attributed to the increased concentration of accumulated gallic acid (Fathy et al., 2018) or the production of carvacrol, thymol, eugenol and caryophyllene oxide (Table 2). Gallic acid was reported to have cytotoxic effects against prostate cancer-3 (PC-3), HeLa and lung A549 cancer cell lines
(Park and Kim, 2013). Also, eugenol has antioxidant and anticancer activities showing chemopreventive properties which are
better than chemically synthesized anticancer drugs. It can induce apoptosis in some cancers including cervical carcinoma,
gastric cancer, melanoma, prostate cancer, skin tumors, osteosarcoma and leukemia (Kong et al., 2014). Moreover, caryophyllene oxide was reported as a potent antioxidant and anticancer which presents cytotoxic activity against
HepG2 and HeLa cancer cell lines (Jun et al., 2011). Caryophyllene oxide has also been reported to have anticancer activity against prostate and breast cancers (Park et al., 2011).
|
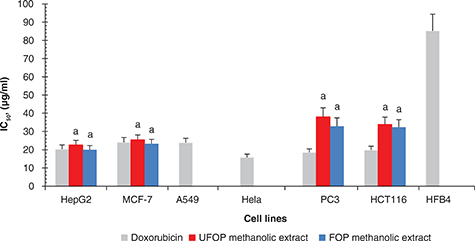 Figure 9. In vitro anticancer activity (IC50, μg/ml) of unfermented (UFOP) and fermented (FOP) olive pomace methanolic extracts in different human cell lines. Figure 9. In vitro anticancer activity (IC50, μg/ml) of unfermented (UFOP) and fermented (FOP) olive pomace methanolic extracts in different human cell lines.
The experiment
was carried out in triplicate. Values are given as mean ± standard error of three batches.
Independent sample t-test was used for comparison of means. a is significantly different from doxorubicin treated group (p < 0.05).
|
|
4. CONCLUSIONSTOP
The SSF of OP by K. marxianus is a new eco-friendly valorization technique that enables the production of valuable bioactive compounds with promising antioxidant
and anticancer activities. This is the first report concerning the effects of FOP extract against different cancer cell lines.
However, few reports have been conducted concerning the anticancer activity of UFOP extracts against different cell lines
which show potent anticancer activity against P815 mastocytoma murine, HepG2 human hepatoma and MDA-MB-231 breast cancer cell
lines. Regarding its high poly phenolic content, OP can be considered as a cheap and renewable source of pharmaceutical compounds
rather than an environmentally polluting agro-industrial waste.
ACKNOWLEDGEMENTTOP
This work was supported by the National Research Centre, Egypt (Project no.11010343).
CONFLICT OF INTERESTTOP
The authors declare that there is no conflict of interest in the study.
REFERENCESTOP
| ○ |
Akpuaka A, Ekwenchi M, Dashak D, Dildar A. 2013. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. NY Sci. J. 6, 119–124.
|
| ○ |
Ali MM, Mahmoud AE, Abdel-Halim AH, Fyiad AA. 2014. Anti-cancer effect of some prepared sulfated oligosaccharides on three
different human cancer cell lines. Asian Journal of Pharmaceutical and Clinical Research 7, 168–176.
|
| ○ |
Aliakbarian B, Casazza AA, Perego P. 2011. Valorization of olive oil solid waste using high pressure–high temperature reactor.
Food Chem. 128, 704–710. https://doi.org/10.1016/j.foodchem.2011.03.092 |
| ○ |
Alu’datt MH, Alli I, Ereifej K, Alhamad M, Al-Tawaha AR, Rababah T. 2010. Optimisation, characterisation and quantification
of phenolic compounds in olive cake. Food Chem. 123, 117–122. https://doi.org/10.1016/j.foodchem.2010.04.011 |
| ○ |
Aly AA, Maraei RW, Ali HG. 2016. Fatty Acids Profile and Chemical Composition of Egyptian Moringa oleifera Seed Oils. J. Am. Oil Chem. Soc. 93, 397–404. https://doi.org/10.1007/s11746-015-2781-6 |
| ○ |
Ciriminna R, Meneguzzo F, Fidalgo A, Ilharco LM, Pagliaro M. 2016. Extraction, benefits and valorization of olive polyphenols.
Eur. J. Lipid Sci. Technol. 118, 503–511. https://doi.org/10.1002/ejlt.201500036 |
| ○ |
Dai J, Mumper RJ. 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352. https://doi.org/10.3390/molecules15107313 |
| ○ |
El Malah T, Nour HF, Nayl A, Elkhashab R, Abdel-Megeid FM, Ali MM. 2016. Anticancer Evaluation of Tris(triazolyl)triazine
Derivatives Generated via Click Chemistry. Aust. J. Chem. 69, 905–910. https://doi.org/10.1071/ch16006 |
| ○ |
Fadel M, El-Ghonemy DH. 2015. Biological fungal treatment of olive cake for better utilization in ruminants nutrition in Egypt.
Int. J. Recycl. Org. Waste Agric. 4, 261–271. https://doi.org/10.1007/s40093-015-0105-3 |
| ○ |
Fathy SA, Mahmoud AE, Rashad MM, Ezz MK, Mohammed AT. 2018. Improving the nutritive value of olive pomace by solid state fermentation
of Kluyveromyces marxianus with simultaneous production of gallic acid. Int. J. Recycl. Org. Waste Agric. 1–7. Available online on 5th Febrauary. https://doi.org/10.1007/s40093-018-0199-5 |
| ○ |
Fonseca GG, Heinzle E, Wittmann C, Gombert AK. 2008. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 79, 339–354. https://doi.org/10.1007/s00253-008-1458-6 |
| ○ |
Hema R, Kumaravel S, Alagusundaram K. 2011. GC/MS determination of bioactive components of Murraya koenigii. J. Am. Sci. 7, 80–83.
|
| ○ |
Ibrahim NM, Yosef HA, Ewies EF, Mahran MR, Ali MM, Mahmoud AE. 2015. Synthesis and Antitumor Evaluation of New Heterocycles
Derived from 3-Methyl-2- benzothiazolinone Hydrazone. J. Braz. Chem. Soc. 26, 1086–1097. https://doi.org/10.5935/0103-5053.20150071 |
| ○ |
Jun NJ, Mosaddik A, Moon JY, Jang K-C, Lee D-S, Ahn KS, Cho SK. 2011. Cytotoxic activity of β - caryophyllene oxide isolated
from Jeju Guava (Psidium cattleianum Sabine) leaf. Rec. Nat. Prod. 5, 242–246.
|
| ○ |
Juntachote T, Berghofer E. 2005. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal.
Food Chem. 92, 193–202. https://doi.org/10.1016/j.foodchem.2004.04.044 |
| ○ |
Khoddami A, Wilkes MA, Roberts TH. 2013. Techniques for analysis of plant phenolic compounds. Molecules 18, 2328–2375. https://doi.org/10.3390/molecules18022328 |
| ○ |
Kong X, Liu X, Li J, Yang Y. 2014. Advances in pharmacological research of eugenol. Curr. Opin. Complement Alternat. Med. 1, 8–11.
|
| ○ |
Kuley E, Durmus M, Balikci E, Ucar Y, Regenstein JM, ÖzoĞul F. 2017. Fish spoilage bacterial growth and their biogenic amine
accumulation: Inhibitory effects of olive by- products. Int. J. Food Prop. 20, 1029–1043. https://doi.org/10.1080/10942912.2016.1193516 |
| ○ |
Lafka T-I, Lazou AE, Sinanoglou VJ, Lazos ES. 2011. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 125, 92- 98. https://doi.org/10.1016/j.foodchem.2010.08.041 |
| ○ |
Mahmoud AE, Ali MM. 2012. Attenuation the side effects of adriamycin-induced cardiotoxicity and nephrotoxicity in rats by
fermented Punica granatum (pomegranate) peel extract. Int. J. Res. Pharm. Sci. 3, 29–37.
|
| ○ |
Mahmoud AE, Fathy SA, Rashad MM, Ezz MK, Mohammed AT. 2018. Purification and characterization of a novel tannase produced
by Kluyveromyces marxianus using olive pomace as solid support, and its promising role in gallic acid production. Int. J. Biol. Macromol. 107, 2342–2350. https://doi.org/10.1016/j.ijbiomac.2017.10.117 |
| ○ |
Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TCd, Coube CS, Leitão SG. 2001. Screening of Brazilian plant extracts for
antioxidant activity by the use of DPPH free radical method. Phytother. Res. 15, 127–130. https://doi.org/10.1002/ptr.687 |
| ○ |
Özkan A, Erdoğan A. 2011. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turkish J. Biol. 35, 735–742.
|
| ○ |
Park, WH, Kim SH. 2013. MAPK inhibitors augment gallic acid-induced A549 lung cancer cell death through the enhancement of
glutathione depletion. Onc. Rep. 30, 513–519. https://doi.org/10.3892/or.2013.2447 |
| ○ |
Park K-R, Nam D, Yun H-M, Lee S-G, Jang H-J, Sethi G, Cho SK, Ahn KS. 2011. β - Caryophyllene oxide inhibits growth and induces
apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 312, 178–188. https://doi.org/10.1016/j.canlet.2011.08.001 |
| ○ |
Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin J-C, Bailleul F, Trotin F. 2000. Phenolic
compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 72, 35–42. https://doi.org/10.1016/s0378-8741(00)00196-3
|
| ○ |
Ramos P, Santos SA, Guerra ÂR, Guerreiro O, Felício L, Jerónimo E, Silvestre AJ, Neto CP, Duarte M. 2013. Valorization of
olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from
olive oil by- products. Ind. Crops Prod. 46, 359–368. https://doi.org/10.1016/j.indcrop.2013.02.020 |
| ○ |
Ranchal I, de Castro M, Muntané J. 2014. Olive Leaves and Mill Waste Exert Anti-Tumoral Properties in Hepatoma Cell Line.
J. Carcinog. Mutagen. 5, 1–10. https://doi.org/10.4172/2157-2518.1000170 |
| ○ |
Schievano A, Adani F, Buessing L, Botto A, Casoliba EN, Rossoni M, Goldfarb JL. 2015. An integrated biorefinery concept for
olive mill waste management: supercritical CO2 extraction and energy recovery. Green Chem. 17, 2874–2887. https://doi.org/10.1039/c5gc00076a |
| ○ |
Shahidi F, Naczk M. 2003. Phenolics in food and nutraceuticals. CRC press.
|
| ○ |
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. 1990. New colorimetric
cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82, 1107–1112. https://doi.org/10.1093/jnci/82.13.1107 |
| ○ |
Suárez M, Romero M-P, Ramo T, Macià A, Motilva M-J. 2009. Methods for preparing phenolic extracts from olive cake for potential
application as food antioxidants. J. Agric. Food Chem. 57, 1463–1472. https://doi.org/10.1021/jf8032254 |
| ○ |
Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. 2011. The peroxisome proliferator-activated receptor: A family of nuclear
receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2, 236–240. https://doi.org/10.4103/2231-4040.90879 |
| ○ |
Uribe E, Lemus-Mondaca R, Vega-Gálvez A, Zamorano M, Quispe-Fuentes I, Pasten A, Di Scala K. 2014. Influence of process temperature
on drying kinetics, physicochemical properties and antioxidant capacity of the olive-waste cake. Food Chem. 147, 170–176. https://doi.org/10.1016/j.foodchem.2013.09.121 |
| ○ |
Wickerham LJ. 1951. Taxonomy of yeasts. US Dept. of Agriculture.
|
| ○ |
Wilkowska A, Kregiel D, Guneser O, Karagul Yuceer Y. 2015. Growth and by-product profiles of Kluyveromyces marxianus cells immobilized in foamed alginate. Yeast 32, 217–225. https://doi.org/10.1002/yea.3044 |
Figure 1. Effect of solvent type on total phenolic recovery from unfermented (UFOP) and fermented (FOP) olive pomace.