Study on the effect of activated carbon with bleaching earth on the reduction of polycyclic aromatic hydrocarbons (PAHs) in bleached soybean oil
N. Aliyar-Zanjania, Z. Piravi-Vanakb,* and M. Ghavamia
aFood Science and Technology Department, College of Food Science and Technology, Science and Research Branch, Islamic Azad University, Tehran, Iran
bFood Industries and Agriculture Research Center, Standard Research Institute, Karaj, Iran
*Corresponding author: zpiravi@gmail.com
| |
SUMMARY
Considering the importance of bleaching earth with activated carbon for reducing the Polycyclic aromatic hydrocarbons (PAHs) as an important chemical contaminant, this study was conducted to confirm the effects of the bleaching process on the reduction or elimination of the BαP index and 4 PAH (BαA, CHR, BβF, BαP) contents in soybean oil. The bleaching process was carried out with different amounts of bleaching earth (1% w/w) and activated carbon (0.1% up to 0.5% w/w). A HPLC/FLD device was employed to determine the PAHs in the oil samples after undergoing extraction and clean-up procedures. The results of linearity indicated that there was a linear response with high linear regression coefficients of determination for all the 4 PAHs analyzed. (R2 > 0.9950). Furthermore, the recovery percentage was calculated from 83.8% to 106.2%; LOD and LOQ were 0.06–0.2 μgkg−1 and 0.2–0.61 μgkg−1, respectively. An analysis of the PAH contents indicated that the bleaching process, including a 0.27% to 0.5% w/w activated carbon application led to the elimination of the PAH content. Since vegetable oils have been shown to be the major sources of PAHs in the diet, the industrial use of activated carbon during the bleaching of vegetable oils is highly recommended.
|
| |
RESUMEN
Estudio sobre el efecto del carbón activo con tierra decolorante sobre la reducción de hidrocarburos aromáticos policíclicos (HAP) en aceite de soja decolorado. Considerando la importancia de decolorar la tierra con carbón activo para reducir los hidrocarburos aromáticos policíclicos (HAP) como un contaminante químico importante, este estudio se realizó para confirmar los efectos del proceso de decolorado sobre la reducción o eliminación del índice BαP y 4 HAP (BαA CHR, BβF, y BαP) en aceite de soja. El proceso de decolorado se realizó con diferentes cantidades de tierra decolorante (1% p/p) y carbón activo (0,1% hasta 0,5% p/p). Se empleó un HPLC/FLD para determinar los HAP en las muestras de aceite después de someterse a procedimientos de extracción y limpieza. Los resultados de linealidad indicaron que hubo una respuesta lineal con altos coeficientes de regresión lineal para los 4 HAP analizados. (R2> 0,9950). Además, el porcentaje de recuperación se calculó de 83,8% a 106,2%; LOD y LOQ fueron 0.06–0.2 μgkg−1 y 0,2–0,61 μgkg−1, respectivamente. El análisis de los contenidos de HAP indicó que el proceso de decoloración que incluían un porcentaje de carbón activo de 0.27% hasta 0.5% p/p llevó a la eliminación del contenido de HAP. Dado que se ha demostrado que los aceites vegetales son las principales fuentes de HAP en la dieta, el uso industrial de carbón activado durante la decoloración de los aceites vegetales es muy recomendable.
|
Abbreviation
| BαP |
Benzo[a]pyrene |
| BαA |
Benz[a]anthracene |
| CHR |
Chrysene |
| BβF |
Benzo[b] Fluoranthene |
| LOD |
Limit of detection |
| LOQ |
Limit of quantification |
| PAHs |
Polycyclic aromatic hydrocarbons |
| HPLC/FLD |
High- performance liquid chromatography with fluorescence detection |
1. INTRODUCTIONTOP
Many crude fats and oils have impurities which should be removed from the oil. These impurities include free fatty acids, phosphatides, waxes, pigments, peroxides, metals and polycyclic aromatic hydrocarbons (PAHs) as well as other impurities. The presence of these impurities in the oil causes darkness, foaming, reduction in smoke point and oxidative stability along with other adverse effects during the process, all of which eventually reduce the safety and quality of the finished product. Therefore, crude oil is supplied as an edible product subjected to processing and the removal of impurities (Yu et al., 2014; Stenerson et al., 2015). Polycyclic aromatic hydrocarbons (PAHs) are among the most toxic compounds in edible oils. Fatty compounds are joined together by a number of aromatic rings, including hydrogen and carbon atoms. These compounds are also semi-volatile or non-volatile, non-degradable, highly organic, stable and toxic for the environment. (Stenerson et al., 2015; Singh et al., 2016).
Mainly due to the incomplete combustion of organic matter during the pyrolysis process (as well as during forest fires, eruption of volcanoes, combustion of wood and fossil fuels, industrial processes and baking of foodstuffs), PAH compounds cause the contamination of oils and fats due to their lipophilic nature. There are two main ways which vegetable oils are contaminated with PAH compounds. The first is environmental contamination, in which soil may be a major source of storage for these compounds in terrestrial environments. On the other hand, contamination through sewage and atmosphere contribute to the concentration of PAH compounds. Therefore, high levels of these compounds are found in edible vegetable oils due to their high levels in the soil and plants of the region. As a result, PAHs may be condensed within plant seeds and, subsequently, into the oil because of their lipophilic nature. The second way is through the direct drying of raw materials (oilseeds) with the combustion of smoke before oil extraction. (CAC/RCP 68., 2009; Camargo et al., 2012; Jiang et al., 2015).
The International Agency for Research on Cancer (IARC), Agency for the Study of Disease and Toxic Substances (ATS-DR) and The United States Environmental Protection Agency (US EPA) have included PAH compounds in the list of primary contaminants, due to their carcinogenicity and mutagenesis. These compounds can become diol-epoxide synergist and interact with macromolecular cells such as DNA, causing errors in the repetition of these molecules and lead to tumors and cancers. According to studies, the average consumption of these compounds is from 0.02 to 3.6 μg per person per day. The maximum level limits of BαP and the four compounds PAH (BαA, CHR, BβF, BαP) in oils and fats used directly or as raw materials are 2 μgkg−1 and 10 μgkg−1, respectively (EC 2011a,b; Akdogan et al., 2016).
The application of a refining process with the goal of reducing the level of PAH compounds can be considered in the steps such as neutralization, bleaching and deodorization. The bleaching step in vegetable oil refining is known as an adsorption process. Among the main factors of the bleaching process, it is possible to refer to bleaching earth with activated carbon. During the bleaching step, in addition to reducing pigments, undesirable compounds, especially PAHs with five and six rings (heavy compounds), are the main pollutants in soybean oil and can be significantly reduced by using activated carbon rather than other refining steps. Soybean oil is one of the most important vegetable oils in the world due to its abundance and low cost. Also, because of the drying process of soybeans, this oil can have a high capacity for PAH formation (Camargo et al., 2012; Vaisali et al., 2015).
There are many studies aimed at determining the levels of PAHs in edible vegetable oils. The sample preparation includes liquid-liquid extraction with C18 SPE cartridges and analysis by the HPLC-FLD. (Camargo et al., 2011; Camargo et al., 2012; Stenerson et al., 2015; Akdogan et al., 2016; Dassilva et al., 2017). In addition, methods such as low temperature clean-up and solid-phase extraction (SPE) (Drabova et al., 2013; Payanan et al., 2013), and modified low- temperature and ultrasound-assisted liquid–liquid extraction method followed by HPLC/FLD have been used for determining the levels of PAHs (Taghvaee et al., 2015a,b, 2016).
Previous literature has been focused on raw or refined vegetable oils to evaluate the amount of PAHs as contamination (Pandey et al., 2004; Payanan et al., 2013; Yu et al., 2014; Jiang et al., 2015; Stenerson et al., 2015; Taghvaee et al., 2015; Akdogan et al., 2016; Krajian et al., 2016; Shi et al., 2016; Dassilva et al., 2017). There is a limited number of studies focused on the effect of oil refining process steps (neutralization, bleaching and deodorization) on the level of PAH compounds (Teixeira et al., 2007; Camargo et al., 2011; Yu et al., 2014). The oil refining technology varies in terms of operation. Iran is a vegetable oil importer, almost none of the oil refineries in Iran uses activated carbon and so far there has been no guidance for its optimal use to remove PAH compounds. Due to the lack of information regarding the proper amount of activated carbon in the bleaching process, this was the main focus of this study, in addition to the effect of the bleaching process on the reduction of PAH compounds. The most important goals of this study include determining of the levels of polycyclic aromatic hydrocarbons BαP and total BαA, CHR, BβF, BαP in bleached soybean oil (with activated carbon and bleaching earth) and the selection of the most suitable level of activated carbon for the purpose of reducing polycyclic aromatic hydrocarbons such as BαA, CHR, BβF, and BαP in soybean oil.
2. MATERIALS AND METHODSTOP
2.1. Reagents and chemicalsTOP
Methanol, n hexane, acetonitrile, acetone, dichloromethane, toluene, water and tetrahydrofuran were purchased from Merck (Darmstadt, Germany), all of which were of HPLC grade.
The standard mixture of the 16 EPA (United States Environmental Protection Agency) PAHs (PAH-mix 4S8743) was purchased from Sigma Aldrich (Bellefonte, PA). The mixture consisted of (acenaphthene, acenaphthylene, anthracene, fluoranthene, fluorine, naphthalene, phenanthrene, pyrene, benz [a] anthracene, benzo [b] fluoranthene, benzo [k] fluoranthene, benzo [ghi] perylene, benzo [a] pyrene, chrysene, dibenz [a,h] anthracene and indeno [1,2,3-cd] pyrene).
The neutralized soybean oil was bought from the Behshahr Company of Iran. The samples were stored at room temperature until analysis.
A bleaching earth (Tonsil 210 optimum) was purchased from the Kanisaz-Jam Company, Iran with the following characteristics: a chemical mixture of bentonite activated with acid; white in color with a powdery appearance; insoluble, with a moisture percentage of 9; free acidity in sulfuric acid of 0.66; density of 500; and pH of 3.
Activated carbon was purchased from Merck (Darmstadt, Germany) with the following characteristics: a chemical mixture of bituminous carbon; 0.5–2 millimeters in size with a diameter of 1.25 millimeters; moisture percentage of 2; and actual density of the particle in kilograms per liter of 0.81; a special surface area per square meter per kilogram of 1.2; the volume of the pores in milliliters per gram of 0.73, with a pore diameter of 2 nanometers.
2.2. InstrumentTOP
An HPLC system (YL 9100 HPLC, South Korea) with binary gradient elution, a solvent reservoir of 1L capacity, a gradient washing program, a solvent storage tank with 1L capacity and rotary filter, a YL 9110 quaternary pump, a YL 9101 vacuum degasser, a sampler and YL 9130 temperature regulator at 25 °C, a fluorescence detector with time schedule for types of propagation waves (FP-2020), computer software for information processing (YL Clarity software program), a Waters C18 reversed-phase column, and a WAT045905 symmetry column reversed-phase 5 μm spherical silica, 4.6 mm 150 mm (Water, Ireland) was used for chromatographic analysis. Florisil-bonded phase cartridges were Chromabond, 3 mL, 500 mg LN 0113/1 (Macherey-Nagel, Germany). The additional equipment included a rotary evaporator (Heidol, Germany), with centrifugation (Dynamc, United Kingdom) at the minimum speed of 4000 rpm, a vortex mixture (Velp Scientifica, Italy), an automatic evaporator (Heraeus, Germany) with nitrogen gas pressure at 34.5 kPa, a scale (Mettler Toledo, Switzerland), an ultrasonic bath (Elma, Germany) and an oven (Heraeus, Germany).
2.3. Bleaching procedureTOP
The bleaching process was not performed on sample a (blank), so it was the same neutralized soybean oil that was injected into the HPLC-FLD device after the process of separating the PAH compounds.
Bleaching of the other neutralized soybean oil samples was done according to the method described by Azadmard and Dutta with slight modifications (Azadmard and Dutta, 2007). Approximately 10 g of the neutralized soybean oil with 1% w/w of bleaching earth and different levels of activated carbon (0.1%, 0.2%, 0.27%, 0.3%, 0.4% and 0.5% w/w) and a standard solution of PAHs at the concentration of 200 μgkg−1 were added to 250 ml round bottom flasks. Then the flasks were attached to the rotary evaporator and mixed at 85 °C. The bleaching process was carried out for 1 h at this temperature under vacuum (1000 mbar) with vigorous stirring of the mixture by the device (100 rpm). After the elapsed time, the mixture was cooled down and immediately filtered on a Buchner funnel under vacuum to remove the bleaching earth and activated carbon. The obtained samples included b1., c2., d3., e4., f5., g6. and h7..
2.4. PAH extraction proceduresTOP
2.4.1. Ultrasound-assisted solvent extraction methodTOP
This method is based on ISO 15753(2006). About 2.5 g of neutralized (blank sample) or bleached soybean oil (sample b, c, d, e, f, g, h) and 10 ml acetone/acetonitrile solvent mixture (60%:40% v/v) in triplicate were extracted and ultrasonicated at 40 °C for 5 min in an ultrasonic bath and then centrifuged (400 rpm/5.0 min). The top layer was separated and evaporated to dryness under a flow of nitrogen. The extract was dissolved in 2 ml of extraction solvent, then mixed again and centrifuged. The top layer was transferred to the C18 cartridge, which was conditioned with 24 ml acetonitrile and 24 ml methanol. The cartridge was eluted with 5 ml of extraction solvent and the extracts were evaporated and dissolved in 1 ml hexane. Afterwards, the extracts were transferred to a Florisil cartridge which was previously conditioned with 15 ml dichloromethane and 12 ml hexane. The cartridge was eluted with 9 ml of a hexane/dichloromethane solvent mixture (75%:25% v/v) and the SPE extracts were evaporated to dryness under a flow of nitrogen up to the volume of 1 ml. Then, 0.5 ml of toluene was added as a holder and the evaporation continued up to the volume of 50 μl. For HPLC-FLD analysis, the residue was dissolved in 250 μL of acetonitrile.
2.5. HPLC-FLD analysisTOP
A standard solution with the concentration of 200 μgkg−1 was prepared for spiking the soybean oil samples. After the extraction and purification of PAH compounds, these compounds were analyzed by YL 9100 HPLC system using a fluorescence detector (HPLC-FLD). Also, five standard solutions of PAHs in acetonitrile with the concentrations of 0, 12.5, 25, 50 and 100 μg·L−1 for the calibration curve were injected into the HPLC-FLD device.
The column temperature of HPLC-FLD was isothermal at 25 °C, injection volume was 20 μL with a flow rate of 0.5 ml·min−1 and a mobile phase acetonitrile (A) and acetonitrile/water, 50:50 (B), were applied. Separation was performed using the gradient elution program on the reverse phase column C18, as described in Table1.
Table 1. Gradient elution program on reverse phase column C18 for HPLC separation, ISO 15753(2006)
| Time (min) |
Solvent mixture A (%) |
Solvent mixture B (%) |
| 0 |
0 |
100 |
| 5 |
0 |
100 |
| 27 |
60 |
40 |
| 36 |
100 |
0 |
| 41 |
100 |
0 |
| 43 |
0 |
100 |
| 45 |
0 |
100 |
For the PAH determination by the fluorescence detector (FLD), the following programmed excitation and emission wavelengths (Ex/Em): were used: 270/385 nm (BαA: CHR) to 18.05 min, 256/446 nm (BβF) to 28 min, 292/410 nm (BαP) to 31.1 min (ISO 15753:
2006).
2.6. Statistical analysisTOP
The data obtained from the results of determining the levels of PAH compounds in the soybean oil samples were analyzed. All the results were the average of three separate experiments. Linear least-square regression equations were used for calibration curves. A factorial experiment was conducted in a completely randomized design. Independent variables included the bleaching earth (1% w/w) and different levels of activated carbon (0.1%, 0.2%, 0.27%, 0.3%, 0.4% and 0.5% w/w) and the studied traits (BαA, CHR, BβF and BαP). The significance of differences among the main effects in the investigated methods was considered by the Duncan test at the significance level of α=1%. For the data analysis, MSTAT-C software (version 2.18, Michigan State University) was employed.
3. RESULTS AND DISCUSSIONTOP
3.1. Ultrasound-assisted solvent extraction (UASE) methodTOP
After passing an extraction and two clean-up steps, the analysis was performed by HPLC with fluorescence detector. Chromatograms of the samples obtained are given in Figure 1 (a, b, c, d, e, f, g and h).
|
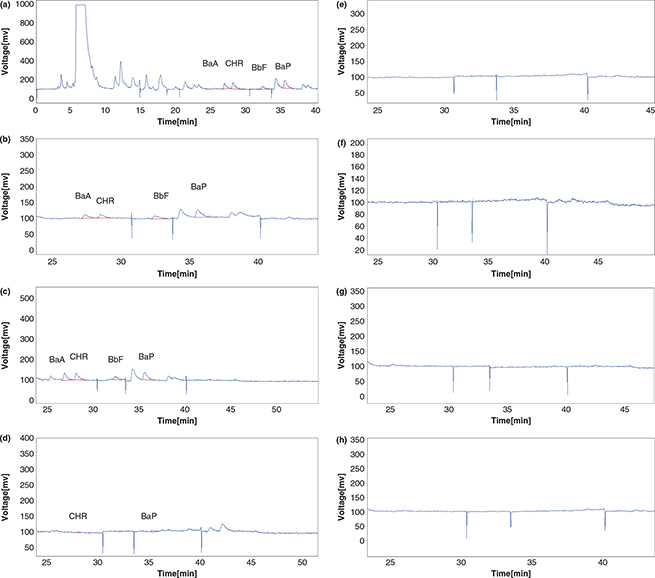 Figure 1. Chromatograms of HPLC-FLD analysis of PAHs after UASE method and two clean-up steps, (each chromatogram was obtained by the injection in triplicate of PAH standard solution at the concentration of 200 μgkg−1). Chromatogram of neutralized soybean oil, blank sample: (a) and chromatograms of samples (b, c, d, e, f, g and h). BaA: Benz[a]anthracene;CHR: Chrysene; BbF: Benzo[b]fluoranthene; BaP:Benzo[a]pyrene Figure 1. Chromatograms of HPLC-FLD analysis of PAHs after UASE method and two clean-up steps, (each chromatogram was obtained by the injection in triplicate of PAH standard solution at the concentration of 200 μgkg−1). Chromatogram of neutralized soybean oil, blank sample: (a) and chromatograms of samples (b, c, d, e, f, g and h). BaA: Benz[a]anthracene;CHR: Chrysene; BbF: Benzo[b]fluoranthene; BaP:Benzo[a]pyrene
|
|
3.2. Method validationTOP
Validation of the method was conducted based on the linearity, repeatability and recovery, limits of detection (LOD) and quantification (LOQ). The linearity of the method was tested separately using a standard curve design for each PAH composition. Standard solutions with the concentrations of 0, 12.5, 25, 50 and 100 μg·L−1prepared for each PAH compound, BαP and Ʃ4PAHs (BαA, CHR, BβF, BαP) were injected with three replications. The mean peak area obtained for each concentration was calculated based on five-point calibration curves. The results indicated that there was a linear response with high linear regression coefficients of determination for all the 4 PAHs analyzed. (R2 > 0.9950), (Table 2). In addition, linear response with high linear regression coefficients of determination for target compounds determined in previous studies was found, with correlation coefficients between 0.9929 and 0.9999. (Rojo Camargo et al., 2012; Tfouni et al., 2014; Jiang et al., 2015; Taghvaee et al., 2015; Akdogan et al., 2016). Accordingly, the developed extraction method provided reasonably good accuracy for the analysis of PAHs in the oil samples and it can be assured that linear data or regression were reliable with a probability of higher than 90% (less than 10% error).
Table 2. Performance criteria of UASE method for the determination of PAHs; LOD, LOQ in μgkg−1, Instrument linearity (R2) and recovery in %
| PAHa |
Linearity R2 |
Recoveryb ± RSD (%)
|
LODc (μgkg−1)
|
LOQd (μgkg−1)
|
| BαA |
0.9991 |
96.85± 5.00 |
0.16 |
0.48 |
| CHR |
0.9951 |
106.24± 4.00 |
0.14 |
0.43 |
| BβF |
0.9985 |
90.43± 3.00 |
0.20 |
0.61 |
| BαP |
0.9988 |
83.84± 3.00 |
0.06 |
0.20 |
a BaA: Benz[a]anthracene;CHR: Chrysene; BbF: Benzo[b]fluoranthene; BaP:Benzo[a]pyrene
b Mean value for two levels 5 and 10 μgkg−1± relative standard deviation (n = 3)
c LOD = 3.3 RSD/slope of calibration curve
d LOQ = 10 RSD/slope of calibration curve |
In order to evaluate the repeatability and the recovery, blank samples of soybean oil were spiked with two levels of all PAHs (5 and 10 μgkg−1). Reproducibility was evaluated by performing three analyses on the same day under the same conditions. The repeatability of the method was expressed as the relative standard deviation (RSD) in %, and recoveries ranged from 83.8% to 106.2% (RSD=3–5%)
(Table 2). According to the Commission Regulation no 835/2011 on the maximum levels for polycyclic aromatic hydrocarbons in foodstuffs, the recovery range is between 50–120% and the results from this study and previous studies were within the limit set for BαP and Ʃ4PAHs. (Teixeira et al., 2007; Camargo et al., 2012; Yu et al., 2014; Taghvaee et al., 2015a,b).
The LOD and LOQ were defined as the lowest analyte concentrations and measured as the detection limit and the quantification threshold, which could generate a signal-to-noise ratio of 3.33 and 10 in the resulting chromatogram. The detection of limit and the quantification of limit were calculated by drawing a five-point standard curve using five different concentrations of PAH (0, 12.5, 25, 50, 100 μg·L−1) and calculating the standard deviations of the standard samples and the calibration curve slope. The results of the detection of limit were in the range of 0.06–0.020 μgkg−1 and the quantification of limit was in the range of 0.20–0.61 μgkg−1 (Table 2). This result complied with the range of LODs and LOQs obtained in previous research. (EC 2011a,b; Camargo et al., 2012; Yu et al., 2014). The results showed adequate sensitivity of the method for the target compounds.
3.3. Determination of PAHs in soybean oil samplesTOP
More than 90% of oil is imported in Iran and contamination levels can vary according to different sources of vegetable oils. Studies have shown that almost none of the oil refineries in Iran use activated carbon for bleaching oils due to its high cost. Therefore, this research is essential for food safety. In this research we tried to find the appropriate level of bleaching earth, especially activated carbon, to reduce or eliminate this contamination and in addition to the determination of the BαP content, Ʃ4PAHs (BαA, CHR, BβF, BαP) were evaluated in bleached soybean oil.
Commission Regulation no. 835/2011a has specified the maximum level of 2.0 μgkg−1 for BαP and 10 μgkg−1 for Ʃ4PAHs in oils and fats as the most suitable indicators of PAHs in food (EC, 2011a,b). In this research the average results obtained from the peak area of each chromatogram, the analyses were done at least in triplicate. Levels of PAH compounds detectable by HPLC_FLD were analyzed in the neutralized and bleached soybean oil samples ranging from 2.2 to 15.1 μgkg−1 (Table 3). Based on the results, the amount of PAH compounds decreased as the result of the bleaching process with bleaching earth and activated carbon. By reducing these compounds by 100% compared to the blank sample and not detecting them by the HPLC-FLD in samples e, f, g and h, none of the target compounds (BαP and Ʃ4PAHs) were observed, indicating the positive effect of bleaching earth and activated carbon on reducing the levels of these compounds. The PAH concentrations in the neutralized soybean oil (blank) and bleached soybean oil samples were significantly different: (P < 1% according to Duncan’s test).
Table 3. PAH contents (μgkg−1) obtained in the analysis of neutralized and bleached soybean oil by HPLC/FLD
| PAH |
Mean concentrations of PAHs (n=3) ± standard deviation in oils |
| aControl sample(a) |
Sample b |
Sample c |
Sample d |
Sample e |
Sample f |
Sample g |
Sample h |
| BαA |
8.32±0.06 |
5.24±0.04 |
2.22±0.02 |
bND |
ND |
ND |
ND |
ND |
| CHR |
15.11±0.12 |
6.72±0.08 |
2.31±0.03 |
c<LOQ(0.43)
|
ND |
ND |
ND |
ND |
| BβF |
7.91±0.08 |
3.61±0.17 |
2.63±0.06 |
ND |
ND |
ND |
ND |
ND |
| BαP |
14.73±0.01 |
6.42±0.07 |
3.94±0.08 |
<LOQ(0.20) |
ND |
ND |
ND |
ND |
BaA: Benz[a]anthracene;CHR: Chrysene; BbF: Benzo[b]fluoranthene; BaP:Benzo[a]pyrene
a Neutralized soybean oil sample
b ND: Not Detectable
c <LOQ: lower than quantification limit |
The results of previous studies (Teixeira et al., 2007; Camargo et al., 2012; Yu et al., 2014), and Codex Alimentarius (CAC/RCP 68, 2009) also show that the use of activated carbon in the bleaching of vegetable oils at the right dosage during the refining process can eliminate PAH contamination and the application of an HACCP system in accordance with the principles and steps as recommended by Codex is one option for reducing PAH compounds.
4. CONCLUSIONSTOP
PAH compounds are known to be important contaminants in food, especially vegetable oils, which can also be considered one of the main sources of PAH in the diet. These compounds cause the contamination of oils and fats due to their lipophilic nature. Among the vegetable oils, soybean oil is one of the most important vegetable oils in the world due to its abundance and low cost, but this oil can have a high capacity for PAH formation because of the drying process it is subjected to. The goals of this study included determining the levels of polycyclic aromatic hydrocarbons BαP and total BαA, CHR, BβF, BαP in bleached soybean oil (with activated carbon and bleaching earth) and the selection of the most suitable level of activated carbon for the purpose of reducing polycyclic aromatic hydrocarbons such as BαA, CHR, BβF, and BαP in soybean oil.
This analytical procedure was based on ISO 15753(2006). One-step ultrasound-assisted solvent extraction and two SPE clean-up steps were used. Then, HPLC/FLD was selected as an applicable and powerful instrumental technique for the PAH analysis.
According to the satisfactory results of the linearity, recovery %, LOD, LOQ, the development of the process of refining edible oils in industrial units, especially with the use of activated carbon in the bleaching step, even in small quantities, due to its ability to reduce or eliminate PAH compounds is essential.
ACKNOWLEDGMENTSTOP
The authors would like to thank the Iranian Oilseed Cultivation and Development Co, and its staff for their cooperation.
FOOTNOTESTOP
1. Bleached soybean oil with 1% w/w of bleaching earth and PAH standard solution
2. Bleached soybean oil with 1% w/w of bleaching earth, 0.1% w/w of activated carbon and PAH standard solution
3. Bleached soybean oil with 1% w/w of bleaching earth, 0.2% w/w of activated carbon and PAH standard solution
4. Bleached soybean oil with 0.27% w/w of activated carbon, PAH standard solution
5. Bleached soybean oil with 1% w/w of bleaching earth, 0.3% w/w of activated carbon and PAH standard solution
6. Bleached soybean oil with 1% w/w of bleaching earth, 0.4% w/w of activated carbon and PAH standard solution
7. Bleached soybean oil with 1% w/w of bleaching earth, 0.5% w/w of activated carbon and PAH standard solution
REFERENCESTOP
| ○ |
Akdogan A, Buttinger G, Wenzl T. 2016. Single-laboratory validation of a saponification method for the determination of four polycyclic aromatic hydrocarbons in edible oils by HPLC-fluorescence detection. Food Additives Contaminants Part A 33, 215–224. https://doi.org/10.1080/19440049.2015.1127430 |
| ○ |
Azadmard-Damirchi S, Dutta PC. 2007. Free and esterified 4, 4′-dimethylsterols in hazelnut oil and their retention during refining processes. J. Am. Oil Chem. Soc. 84, 297–304. https://doi.org/10.1007/s11746-006-1025-1 |
| ○ |
Camargo MCR, Antoniolli PR, Vicente E. 2011. Polycyclic aromatic hydrocarbons in Brazilian commercial soybean oils and dietary exposure. Food Additives Contaminants Part B 4, 152–159. https://doi.org/10.1080/19393210.2011.585244 |
| ○ |
Camargo MCR, Antoniolli PR, Vicente E. 2012. Evaluation of polycyclic aromatic hydrocarbons content in different stages of soybean oils processing. Food Chem. 135, 937–942. https://doi.org/10.1016/j.foodchem.2012.06.031 |
| ○ |
Codex alimentarius developed a code of practice for the reduction of contamination of food with PAHs from smoking and direct drying processes (CAC/RCP 68, 2009). |
| ○ |
Dassilva SA, Sampaio GR, Dasilva Torres EAF. 2017. Optimization and validation of a method using UHPLC-fluorescence for the analysis of polycyclic aromatic hydrocarbons in cold-pressed vegetable oils. Food Chem. 221, 809–814. https://doi.org/10.1016/j.foodchem.2016.11.098 |
| ○ |
Domingo JL, Nadal M. 2015. Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Food Chem. Tox. 86, 144–153. https://doi.org/10.1016/j.fct.2015.10.002 |
| ○ |
Drabov L, Tomaniova M, Kalachova K, Kocourek V. 2013. Application of solid phase extraction and two-dimensional gas chromatography coupled with time-of-flight mass spectrometry for fast analysis of polycyclic aromatic hydrocarbons in vegetable oils. Food Control 33, 489–497. https://doi.org/10.1016/j.foodcont.2013.03.018 |
| ○ |
European Commission EC. 2011a. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Official Journal of European Commission 215, 7–8. |
| ○ |
European Commission EC. 2011b. Commission Regulation (EU) No836/2011 of 19 August 2011 amending regulation(EC) No 333/2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo (a) pyrene in foodstuffs. Official Journal of the European Union 215, 9–16. |
| ○ |
ISO Method(2006)15753. in International Standard Methods for Animal and vegetable fats and oils. Determination of polycyclic aromatic hydrocarbons. ISO 15753. |
| ○ |
Jiang D, Xin C, Li W, Chen J, Li F, Chu Z, Xiao P, Shao L. 2015. Quantitative analysis and health risk assessment of polycyclic aromatic hydrocarbons in edible vegetable oils marketed in Shandong of China. Food Chem. Tox. 83, 61–67. https://doi.org/10.1016/j.fct.2015.06.001 |
| ○ |
Krajian H, Odeh A. 2016. Levels of 15 + 1 EU priority polycyclic aromatic hydrocarbons in different edible oils available on the Syrian market. Polycyclic Aromatic Compounds https://doi.org/10.1080/10406638.2016.1220958 |
| ○ |
Lacoste F. 2014. Undesirable substances in vegetable oils: anything to declare?. Oilseeds and fats, Crops Lipid 21, A10. https://doi.org/10.1051/ocl/2013060 |
| ○ |
Pandey M, Mishra K, Khanna S. 2004. Detection of polycyclic aromatic hydrocarbons in commonly consumed edible oils and their likely intake in the Indian population. J. Am. Oil Chem. Soc. https://doi.org/10.1007/s11746-004-1030-4 |
| ○ |
PayananT, Leepipatpiboon N, Varanusupakul P. 2013. Low-temperature cleanup with solid-phase extraction for the determination of polycyclic aromatic hydrocarbons in edible oils by reversed phase liquid chromatography with fluorescence detection. Food Chem. 141, 2720–2726. https://doi.org/10.1016/j.foodchem.2013.05.092 |
| ○ |
Shi L, Liu Y, Zhang D. 2016. Incidence and survey of polycyclic aromatic hydrocarbons in edible vegetable oils in China. Food Control 62, 165–170. https://doi.org/10.1016/j.foodcont.2015.10.037 |
| ○ |
Singh L, Varshney JG, Agarwal T. 2016. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 199, 768–781. https://doi.org/10.1016/j.foodchem.2015.12.074 |
| ○ |
Stenerson K, Shimelis O, Halpenny MR, Espenschied K, Ye MM. 2015. Analysis of Polynuclear Aromatic Hydrocarbons in Olive Oil after Solid-Phase Extraction Using aDual-Layer Sorbent Cartridge Followed by High-Performance Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 63, 4933–4939. https://doi.org/10.1021/jf506299f |
| ○ |
Taghvaee Z, Piravivanak Z, Rezaee K, Faraji M, Nanvazadeh S. 2015a. The potential of low temperature extraction method for analysis of polycyclic aromatic hydrocarbons in refined olive and refined pomace olive oils by HPLC/FLD. Nut. Food Sci. Res. 2, 47–54. http://nfsr.sbmu.ac.ir/article-1-104-fa.html |
| ○ |
Taghvaee Z, Piravivanak Z, Rezaee K, Faraji M. 2015b. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Olive and Refined Pomace Olive Oils with Modified Low Temperature and Ultrasound-Assisted Liquid–Liquid Extraction Method Followed by the HPLC/FLD. Food Anal. Methods 9, 1220–1227. https://doi.org/10.1007/s12161-015-0297-1 |
| ○ |
Taghvaee Z, Piravivanak Z, Rezaee K, Faraji M. 2016. Determination of polycyclic aromatic hydrocarbons in olive oil and refined pomace olive oils HPLC/FLD. J. Food Biosci. Technol. 6, 77–85. http://jfbt.srbiau.ac.ir/article_8914_1321.html |
| ○ |
Teixeira H, Oliveira M, Casal S. 2007. PAHs content in sunflower, soybean and virgin olive oils: Evaluation in commercial samples and during refining process. Food Chem. 104, 106–112. https://doi.org/10.1016/j.foodchem.2006.11.007 |
| ○ |
Vaisali C, Charanyaa S, Belur PD, Regupathi I. 2015. Refining of edible oils: a critical appraisal of current and potential technologies. Internat. J. Food Sci. Techn. 50, 13–23. https://doi.org/10.1111/ijfs.12657 |
| ○ |
Yu Y, Wang Y, Jin Q, Dong H, Wang X. 2014. Sources of Polycyclic Aromatic Hydrocarbons in Soybean Oil and its Dynamic Changes Refining Processing. Adv. J. Food Sci. Techn. 6, 2. https://doi.org/10.19026/ajfst.6.3028 |
Figure 1. Chromatograms of HPLC-FLD analysis of PAHs after UASE method and two clean-up steps, (each chromatogram was obtained by the injection in triplicate of PAH standard solution at the concentration of 200 μgkg−1). Chromatogram of neutralized soybean oil, blank sample: (a) and chromatograms of samples (b, c, d, e, f, g and h). BaA: Benz[a]anthracene;CHR: Chrysene; BbF: Benzo[b]fluoranthene; BaP:Benzo[a]pyrene