Biochemical, compositional, and spectral analyses of İsot (Urfa pepper) seed oil and evaluation of its functional characteristics
B. Başyiğita, Ş. Dağhana and M. Karaaslana, *
aHarran University, Engineering Faculty, Food Engineering Department, Şanlıurfa, Turkey.
*Corresponding author: mk385@cornell.edu
| |
SUMMARY
In this study, the physicochemical, functional, and antimicrobial properties of pepper seed oil (PSO) were determined. PSO was subjected to differential scanning calorimeter (DSC), fatty acid composition, carotenoid, capsaicin, and tocopherol analyses. LC-ESI-MS/MS and NMR were used to characterize and quantify phytochemicals. Resveratrol, luteolin, and 4-hydroxycinnamic acid were the principal phenolics in PSO. A high concentration of unsaturated fatty acids (85.3%), especially linoleic acid (73.7%) is present in PSO. Capsaicin, dihydrocapsaicin, α-tocopherol, δ-tocopherol, zeaxanthin, and capsanthin were determined in PSO at concentrations of 762.92, 725.73, 62.40, 643.23, 29.51, 16.83 ppm, respectively. PSO displayed inhibitory activity against α-glucosidase rather than α-amylase. The antimicrobial activity of PSO was tested against Escherichia coli, Staphylococcus aureus subsp. aureus, Aspergillus brasiliensis and Candida albicans. The antimicrobial potential of PSO was expressed as minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and inhibition zone (IZ) diameter. Polyunsaturated fatty acid, capsaicin, carotenoid, tocopherol, resveratrol contents; the antioxidant, α-glucosidase inhibitory and antimicrobial activities of PSO indicated its nutritional value and health promoting nature for the well-being of humans.
|
| |
RESUMEN
Análisis bioquímico, composicional y espectral del aceite de semilla de isot (pimiento Urfa) y evaluación de sus características funcionales. En este estudio se determinaron las propiedades fisicoquímicas, funcionales y antimicrobianas del aceite de semilla de pimiento (ASP). El ASP se sometió a análisis de calorimetría diferencial de barrido (DSC), composición en ácidos grasos, carotenoides, capsaicina y tocoferoles. LC-ESI-MS / MS y RMN se usaron para caracterizar y cuantificar fitoquímicos. El resveratrol, la luteolina y el ácido 4-hidroxicinámico fueron los principales compuestos fenólicos en el ASP. Una alta concentración de ácidos grasos insaturados (85,3%), especialmente ácido linoleico (73,7%) están presentes en el ASP. Se determinaron capsaicina, dihidrocapsaicina, α-tocoferol, δ-tocoferol, zeaxantina y capsantina en el ASP en concentraciones de 762.92, 725.73, 62.40, 643.23, 29.51, 16.83 ppm, respectivamente. ASP mostró actividad inhibitoria contra la α-glucosidasa en lugar de la α-amilasa. La actividad antimicrobiana de ASP se probó contra Escherichia coli, Staphylococcus aureus subsp. aureus, Aspergillus brasiliensis y Candida albicans. El potencial antimicrobiano de ASP se expresó como concentración inhibitoria mínima (MIC), concentración bactericida mínima (MBC) y diámetro de la zona de inhibición (IZ). Los contenidos de ácidos grasos insaturados, capsaicina, carotenoide, tocoferol, resveratrol; las actividades antioxidantes, inhibidoras de la α-glucosidasa y antimicrobianas del ASP indicaron su valor nutritivo y la naturaleza promotora de la salud y el bienestar humano.
|
1. INTRODUCTIONTOP
The genus Capsicum belongs to the family Solanaceae and includes at least 27 species of annual and perennial herbs or shrubs that are native to Central and South America (Walsh and Hoot, 2001). Capsicum annum L., Capsicum baccatum L., Capsicum chinense Jacq., Capsicum frutescens L. and C. pubescens Ruiz and Pav. are cultivated species in the Capsicum genus. Among these, C. annum – the common bell pepper – accounts for most commercial production worldwide (Jarret et al., 2013). However, numerous other forms of these species are also grown on a commercial scale. These would include: Cayenne, Guindilla Verde, Scotch Bonnet Pepper, Basque Fryer, Jalapeño, Paprika, Anaheim, İsot pepper and many other less commonly known locally grown ones. In the last decade, the worldwide pepper production continued to increase and reached 38.4 x 106 MT in 2014. Peppers are widely cultivated in China, and Turkey, as well as Mexico. Turkey is the third largest pepper producer in the world. The most famous pepper variety cultivated in the South East region of Anatolia is called ‘İsot’. The İsot pepper is generally consumed in dried powder form. After harvest, fresh peppers are sliced, dried and ground into a powder to produce İsot pepper spice. Urfa cuisine is famous for using İsot pepper flakes in dishes, salads, and sauces. It is often described as having a smoky, bitter-sweet taste due to its rich capsaicin and sugar content. During the processing of İsot peppers into spices; seeds, stems and placenta are removed as by-products (Yılmaz et al., 2015). Fruit and vegetable wastes are generally being used as livestock feed and sometimes as raw materials for the manufacturing of other added-value products. By 2050 the world is going to be feeding 2 billion more people and will possibly require additional sources for the production of foods or food ingredients. An ample amount of fruit and vegetable wastes and by-products from the food processing industry are available throughout the world. For most of the pepper varieties, seeds account for almost 20% of the dry-weight of the whole fruit (Bosland and Votava, 2000). During the industrial processing of peppers, the seeds are generally removed as waste, even though they contain a large amount of biologically active compounds with anti-inflammatory, and antioxidant properties (Di Sotto et al., 2018; Sandoval-Castro et al., 2017). Pepper seeds are composed of fibers, carbohydrates, proteins, lipids, and some micronutrients including phenolic acids, flavonoids, vitamins and pigments. Pepper seeds represent a rich source of nutritive macromolecules. Pepper seeds are widely used by small, landless farmers as livestock feed or adulterated into spices to make more profit. The oil content of pepper seeds is above 20% and the characteristic properties of seed oil should be illuminated for processing into added-value products. Possible industrial uses of pepper (Capsicum annum L.) derived products and their technological benefits and biological advantages were recently reviewed (Baenas et al., 2019). The evaluation of plant wastes to develop novel added-value products is important in both economic and environmental aspects. Nowadays, the demand for pepper seed oil is increasing and large quantities of pepper seed oil is transported to Europe and the USA. The use of fruit and vegetable waste extracts including oils has become a significant issue in order to develop novel added-value products. Therefore, it is necessary to characterize the oil extracted from İsot pepper seeds grown in the southeastern region of Anatolia. In this study, İsot pepper seeds were collected from the şanlıurfa region and used for extracting oil by the cold-press method. The biochemical, thermal, compositional, and functional characteristics of İsot (Urfa pepper) seed oil were determined; and its antimicrobial activity on pathogenic microorganisms was assessed in the context of the study.
2. MATERIALS AND METHODSTOP
2.1. Plant materialsTOP
Seeds were obtained from a local pepper grower in şanlıurfa, Turkey. The seeds were dried on trays refrained from sunlight at room temperature for 7 days and stored at 4 ºC until used in the experiments. The chemicals and standards were obtained either from Sigma or Merck (Darmstadt, Germany) unless otherwise stated.
2.2. Pepper seed oil extractionTOP
PSO extraction from İsot pepper seeds was carried out using a laboratory scale cold pressing machine (12 kg seed/h capacity, single head, 750 W power). Centrifugation (1420 g, 10 °C) and filtration were applied to obtain pure oil. The resulting supernatant was collected and stored in amber-colored vials at -20 ºC after flushing with nitrogen until analysis. 10 g of oil were mixed with 2 mL of methanol (X2) in a vial to extract PSO phenolics. After vortexing, the mixture was centrifuged at 1420 g for 30 min and then the resulting supernatant was used for total phenolic content, total flavonoid content, antioxidant activity, antimicrobial activity, enzyme inhibition activity, NMR, and LC-ESI-MS/MS analyses.
2.3. Physicochemical analysesTOP
Physicochemical analyses (peroxide value, iodine number, free fatty acid (FFA), color (L*, a* and b*), saponification, refractive index, acid value) (AOAC, 1984), total phenolic content (Chen and Young-Hwa, 2013), total flavonoid content (Zhishen et al., 1999), four different antioxidant activity methods: DPPH and ABTS (Çam et al., 2009), CUPRAC (Apak et al., 2007), FRAP (Benzie and Strain, 1996) assays and enzyme (α-glucosidase and α-amylase) (McDougall et al., 2005) inhibition activity were determined accordingly.
2.4. Antimicrobial activityTOP
PSO antimicrobial activity was tested against two reference bacterial strains, one yeast and one mold including Staphylococcus aureus subsp. aureus (ATCC 6538P), Escherichia coli (ATCC 8739), Aspergillus brasiliensis (ATCC 16404) and Candida albicans (ATCC 29212). The agar-well diffusion method was employed for the evaluation of PSO antimicrobial activity. 9-mm holes were punched into the solidified agar and these holes were filled with 120 μl of PSO extract at 200 mg/mL concentration. The microbial count was set at 0.5 Mcfarland standard equivalent to give a concentration of 1*107 bacteria, 1*105 yeast, 1*105 mold per milliliter. The bacteria, yeast and mold cultures were grown in the plates and incubated at 37 °C for 24 h, 25 °C for 24 h, and 25 °C for 5 days, respectively. For the determination of inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), the PSO extracts were diluted in the range of 6.25 mg/mL to 200 mg/mL.
2.5. HPLC analysesTOP
2.5.1. Sample preparation for PSO tocopherol, carotenoid, and capsaicinoid contentsTOP
Prior to tocopherol and carotenoid analyses, the PSO was saponified to separate the triacylglycerides (Fromm et al., 2012). Capsaicinoid extraction from PSO was evaluated by the method described previously (Collins et al., 1995).
2.5.2. Tocopherol contentTOP
An HPLC system (Agilent 1100 series, Agilent, USA) was used to determine the tocopherol fraction. Chromatographic separations were carried out on a Lichrosorb SI60 column (250 x 4 mm, particle size 5 μm) using hexan/2-propanol as the mobile phases, fluorescence detector (Ex 290 nm and Em 330 nm) at a flow rate of 1 mL/min and injection volume of 20 μL (Pinheiro-Sant’Ana et al., 2011).
2.5.3. Carotenoid contentTOP
The PSO carotenoid fractions were determined by a Purospher® STAR RP-18 column (250x4.6 mm particle size 5 μm) at 31 ºC with a Shimadzu prominence HPLC System (Shimadzu, Japan) using acetone (A) and water (B) as the mobile phases at a flow rate of 1.25 mL/min and injection volume of 20 μL. The gradient elution program was as follows: 25% B for 5 min, 20% B for 10 min, 5% B for 15 min, 100% A for 23 min and back to the initial conditions of 25% B. The UV-Vis detector was adjusted to 450 nm.
2.5.4. Capsaicinoid contentTOP
PSO extracts were injected to a HPLC system (Shimadzu, Japan) with an UV-Vis detector and a column ODS-3 (250 x 4.6 mm particle size 5 μm) at 40 ºC using solvent A (1% acetic acid) and solvent B (acetonitrile) as the mobile phases at a flow rate of 1 mL/min and injection volume of 20 μL. The gradient conditions were: 100% A for 15 min, 100% B for 20 min, 100% A for 25 min and back to the initial conditions of 100% A. The absorbance was read at 280 nm.
2.6. Fatty acid profileTOP
The fatty acid composition of PSO was analyzed by a gas chromatography (GC-FID, Shimadzu, GCMSQP2010, JAPAN) with a fused-silica capillary column (30 m 0.25 mm) coated with 0.25 mm dimethyl polysiloxane (RTX®-5MS, Restek). The column temperature was programmed from 40 °C (held for 2 min) to 240 °C at 3 °C/min increases. Injector, interface and detector temperatures were 250, 250 and 220 °C, respectively. Helium was used as carrier gas (1.0 mL/ min.) at a split ratio of 1:20.
2.7. LC-ESI-MS/MS analysis of phenolic compoundsTOP
PSO extracts were filtered through a 0.45 μm pore size membrane filter and then injected into Nexera Shimadzu UHPLC LC–ESI-MS/MS (Shimadzu, Japan) equipped with a binary gradient pump, an autosampler (SIL-20AC), a degasser (DGU-20A3R) and a column thermostat (CTO-10ASVP). The column was an Intersil ODS-4 analytical column with 3 mm i.d. x 100 mm and 2μm particle size. Experiments to elute phenolic compounds were conducted based on the following conditions: 0.3 mL min-1 flow rate, 40 °C column temperature and 2 μL injection volume, using a mixture of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol) as mobile phase; gradient conditions: from 0 to 4 min 5% B, from 4 to 7 min 95% B, from 7 to 7.01 min 95% B and at 7.01 min back to the initial conditions of 5% B. The concentrations of phenolic compounds are expressed in μg/kg units.
2.8. Nuclear magnetic resonance spectroscopy (NMR)TOP
NMR experiments were conducted on an Agilent Instrument NMR Spectrometer operating at 400 MHz. The PSO was dissolved in chloroform and placed into 5 mm NMR tubes, and injected into the NMR spectrometer to obtain 1H NMR spectra. The acquisition parameters were: spectral width, 6410.3 Hz; spin: 20; number of scans 32; relaxion delay 5s; pulse angle 90°; observe pulse 8.50 μs for 1H NMR spectra.
2.9. Thermal analysis by DSCTOP
For the thermal parameters, a Shimadzu DSC-60 differential scanning calorimeter (DSC) (Shimadzu, Kyoto, Japan) was used. The DSC instrument was calibrated with indium (heat of fusion 28.45 J/g). Temperature calibration was carried out using hexane (m.p 93.5 °C), water (m.p. 0.0 °C) and indium (m.p. 156.6 °C). Samples were weighed into aluminum pans at 10–15 mg and covers were hermetically sealed into place. An empty pan was used for reference. Samples were heated to 60 °C under nitrogen flow (0.5 mL/min), held for 10 min at constant temperature and cooled to -60 °C, and then heated again to 60 °C. The scanning rate was 2 °C/min. The machine equipment program (TA-60WS) was used to plot and analyze the thermal data (Calligaris et al., 2004).
2.10. Data processingTOP
The PSO extracted from three different seeds of the same origin was used for each analysis and all analyses within each PSO were carried out at least in duplicate. Origin Pro 8 (Origin Lab Inc.) and Lab Solutions software (Shimadzu) was used to obtain DSC graphs. The differences among data were compared with independent samples according to the t-test and One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test at the confidence level of 95% (p ≤ 0.05) using SPSS 22.0.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA).
3. RESULTS AND DISCUSSIONTOP
3.1. Physicochemical properties of the İsot seed oilTOP
The physicochemical characteristics of İsot seed oil are given in Table 1. All three color parameters for PSO were higher compared to capia pepper seed oil (Yılmaz et al., 2015) and its color was a deeper reddish and yellowish than that extracted from seed of capia. There is no internationally accepted PSO standard defined in the codex alimentarius. Therefore, we compared the obtained data regarding PSO physicochemical properties with the virgin olive oil standards published in codex alimentarius. The FFA content of PSO (0.22%) was below 0.8%, which was required for an olive oil to be included in the extra virgin category according to the International Olive Oil Standards. The FFA of pepper seed oil for California Wonder (C. annuum) and Aji Colorado were 0.71 and 4.74% (Jarret et al., 2013), respectively, both higher compared to what we measured for İsot PSO. The peroxide value of PSO was (6.35 meq O2/kg), below 20 meq O2/kg, which meets the criteria for high quality olive oils. Saponification value was slightly lower and Iodide value was slightly higher for PSO when compared to standards, as determined by 181.45 and 94.26, respectively (Table 1).
Table 1. Physicochemical properties of pepper seed oil
| Physicochemical properties |
Values |
| Iodide value |
94.26±1.00 |
| Peroxide value (meq O2/kg) |
6.35±0.63 |
| Free fatty acid (%) |
0.22±0.01 |
| Saponification value |
181.45±1.16 |
| Color (L, a*, b*) |
35.92±0.02; 20.40±0.05; 18.24±0.14 |
| Refractive index (24 °C) |
1.45±0.01 |
| Density (g/mL) |
0.91±0.02 |
| Specific gravity |
0.92±0.02 |
| Energy value (kcal/g) |
9.00±0.04 |
| Each value is expressed as a mean ± standard deviation of three replicates (n = 3). |
3.2. Capsaicin, tocopherol, and carotenoid contents of İsot seed oilTOP
Capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin are members of the capsaicinoid family. Capsaicin and dihydrocapsaicin represent 80-90% of the total capsaicinoids present in the capsicum genus (Figueiredo et al., 2013). The ratio of capsaicin/dihydrocapsaicin ranges from 1:1 to 2:1 (Hayman and Kam, 2008). The mean concentrations of capsaicin and dihydrocapsaicin in red chili pepper are 309.3 ppm and 238.2 ppm and 138.5 ppm and 146.4 ppm in green chili pepper (Othman et al., 2011). The capsaicin and dihydrocapsaicin contents of PSO were 762.92 ppm and 725.73 ppm (Table 2), a higher concentration compared to those of red chili pepper and lower than hot chilies. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a unique alkoloid found primarily in pepper fruits with analgesic effects, which give them their hot taste and spicy flavor. Capsaicin exerts selective effects on the peripheral part of the sensory nervous system and relieves pain by depleting the neurotransmitter of painful impulses known as substance P from the sensory nerve terminals. The main source of capsaicinoids in nature is Capsicum fruits. The capsaicin content of peppers is closely linked to their commercial quality and pharmaceutical properties. Tocopherols are considered lipophilic antioxidants, which belong to the Vitamin E group of natural compounds. Tocopherols are found in distinct plant tissues such as fruits, flowers and seeds. Peppers contain tocopherols in amounts comparable to those in spinach and asparagus; while higher than tomatoes (Bosland and Votava, 2000). The PSO evaluated in this study included both α (62.40 ± 0.85 ppm) and δ tocopherols (643.23 ± 1.40 ppm) (Table 2). A group of carotenoids including violaxanthin, capsanthin, crytocapsin, zeaxanthin, β-apo-8’carotenal, cryptoxanthin, and β-carotene was detected in PSO. The unique keto carotenoids capsanthin and cryptocapsin provide the brilliant red color; while β-carotene, zeaxanthin, and violaxanthin impart the yellow-orange color. β-apo-8’carotenal is an aldehydic carotenoid which is widely distributed in peppers and has an orange-red color. Besides their use as natural colorants carotenoids promote human health due to their nutritional characteristics and preventive-protective roles against degenerative diseases (Arimboor et al., 2014). Statistically significant differences (p < 0.05) were found among the carotenoid fractions of PSO and zeaxanthin was the principal carotenoid (29.51 ± 2.00 mg/kg) in PSO. The phenolic and flavonoid contents of the PSO samples are shown in Table 2. Total phenolic content of PSO was higher than that of total flavonoids (p < 0.05). The concentration of total phenolic compounds was 201.84 ± 2.03 (mg GAE/kg) and the flavonoid content was 164.80 ± 1.56 (mg QE/kg). Total phenolic content of İsot seed oil was higher compared to that of capia peppers (Yılmaz et al., 2015). The capsaicin, tocopherol, phenolic and flavonoid contents together with the remarkable antioxidant activity of PSO reveal its rich nutraceutical value and importance for human nutrition.
Table 2. Some bioactive compounds in pepper seed oil
| Capsaicinoids (mg/kg) |
|
Tocopherols (mg/kg) |
|
Carotoneoids (mg/kg) |
|
Phenolics (mg GAE/kg-mg QE/kg) |
| Capsaicin |
762.92±1.51* |
α Tocopherol |
62.40±0.91 |
Violaxanthin |
0.90±0.00 |
Total Phenolics 201.84±2.03* |
| Dihydrocapsaicin |
725.73±1.42 |
δ Tocopherol |
643.23±1.40* |
Capsanthin |
16.83±1.10 |
Flavonoids 164.80±1.61 |
| |
|
|
|
Zeaxanthin |
29.51±2.00* |
- |
| |
|
|
|
β-apo-8’-carotenal |
0.31±0.00 |
- |
| |
|
|
|
Cryptoxanthin |
12.74±0.73 |
- |
| |
|
|
|
β-Karoten |
6.61±0.52 |
- |
| Each value is expressed as a mean ± standard deviation of three replicates (n = 3). Statistically significant differences among results in the same column at the confidence level of 95% for each parameter are marked with *. GAE: Gallic acid equivalents, QE: Quercetin equivalent. |
3.3. Fatty acid composition of İsot seed oilTOP
The fatty acid content of PSO is shown in Table 3. The extracted PSO was mainly composed of unsaturated fatty acids (UFA), approximately 85.3%, including mono-unsaturated fatty acids (oleic) and the poly-unsaturated fatty acid (linoleic). The saturated fatty acids (SFA; palmitic and stearic acids) were also present in PSO but at lower concentrations (14.7%). These results are in agreement with previously published reports (Yılmaz et al., 2015). The ratio of UFA to SFA was 5.8, which was higher than previously reported (Chougui et al., 2013). Linoleic acid was the dominating fatty acid (73.77%) followed by palmitic acid (10.86%) and oleic acid (10.51%). The percentage of linoleic acid (C18:2) found in İsot pepper seed was higher than that found in Capsicum galapagoense, similar to Capsicum flexuosum and lower than Capsicum tovarii (Jarret et al., 2013). The observed differences in the fatty acid composition and percentages of PSO could be variety-specific or due to environmental factors and agronomic practices. The essential fatty acids were named as f-vitamins and draw significant attention due to the health-promoting potential of polyunsaturated fatty acids. It has been reported that unsaturated fatty acids play a role in preventing cardiovascular diseases, diabetes, cancer, Alzheimer’s disease and the alleviation of certain chronic defects (Shahidi and Ambigaipalan, 2018).
Table 3. Fatty acid contents in pepper seed oil
| Fatty acids |
Retention Time |
Content (%) |
| Myristic acid (C14:0) |
26.84 |
0.17±0.01 |
| Pentadecanoic acid (C15:0) |
28.73 |
0.02±0.00 |
| Palmitic acid (C16:0) |
30.59 |
10.86±0.68 |
| Palmitoleic acid (C16:1) |
31.79 |
0.29±0.01 |
| Stearic acid (C18:0) |
34.05 |
3.33±0.20 |
| Oleic acid (C18:1n9c) |
35.11 |
10.51±0.60 |
| Linoleic acid (C18:2n6c) |
36.82 |
73.77±1.50 |
| Arachidic acid (C20:0) |
37.27 |
0.20±0.02 |
| Cis-11-eicosenoic acid (C20:1) |
38.31 |
0.08±0.00 |
| Linolenic acid (C18:3n6) |
38.55 |
0.25±0.01 |
| Cis-11,14-eicosadienoic acid (C20:2) |
39.92 |
0.18±0.01 |
| Cis-13,16-docosadienoic acid (C22:2) |
43.99 |
0.12±0.02 |
| Each value is expressed as a mean ± standard deviation of three replicates (n = 3). |
3.4. Phenolic composition of İsot seed oilTOP
There is limited study in the literature on the phenolic composition of Capsicum annuum L. seed oils. However, the phenolic compositions of peppers have been studied (Baenas et al., 2019). Phenolic compounds belong to phenolic acid derivatives and flavonoids represent major secondary metabolites in peppers (Jayaprakasha et al., 2012). Quercetin, luteolin, myricetin, kaempferol, and apigenin are present in pepper fruits (Asnin and Park, 2015; Baenas et al., 2019; Jayaprakasha et al., 2012). In addition to these compounds, phenolic acids such as caffeic, p-coumaric, sinapic, and ferulic acid are found in peppers (Materska and Perucka, 2005). In this study, the phenolic compounds in PSO were determined using a LC-ESI-MS/MS method. Fourteen phenolic compounds were detected in PSO and resveratrol (6269.10μg/kg) was the most abundant one, followed by luteolin (733.81μg/kg) and 4-hydroxycinnamic acid (517.90μg/kg) (Table 4). Resveratrol has curing effects on stress and disease in rodent models (Baur and Sinclair, 2006); luteolin significantly inhibits neuronal cell apoptosis, elevates cell viability, and attenuates diabetes-associated disorders (Liu et al., 2017); and hydroxycinnamic acid and their derivatives are important components of medicinal herbs which are useful for treatment of chronic diseases (El-Seedi et al., 2018). The synergistic activity of these compounds and vitamin C and vitamin E are responsible for the potent antioxidant capacity of peppers which is associated to the protection of the human body against arteriosclerosis, osteoporosis, diabetes, cancer, and coronary diseases (Pandey and Rizvi, 2009).
Table 4. Phenolic composition of pepper seed oil
| Phenolic compound |
RT |
MF |
Concentration (μg/kg) |
Precursor Ion |
| Fumaric acid |
3.674 |
C4H4O4 |
54.64±1.58 |
115.20 |
| Gallic acid |
4.134 |
C7H6O5 |
48.61±1.05 |
169.20 |
| Catechin |
4.958 |
C15H14O6 |
25.43±1.76 |
291.10 |
| Caffeic acid |
5.283 |
C9H8O4 |
10.12±0.76 |
179.20 |
| Resveratrol |
5.713 |
C14H12O3 |
6269.10±5.65 |
229.10 |
| 4-Hydroxycinnamic acid |
5.738 |
C9H8O3 |
517.90±1.01 |
163.20 |
| Vanillic acid |
6.026 |
C8H8O4 |
108.32±2.24 |
168.80 |
| Butein |
6.084 |
C15H12O5 |
17.91±1.20 |
271.10 |
| Quercetin |
6.091 |
C15H10O7 |
62.71±0.29 |
301.10 |
| Naringenin |
6.104 |
C15H12O5 |
126.61±1.27 |
271.10 |
| Salicylic acid |
6.104 |
C7H6O3 |
52.62±1.02 |
137.20 |
| 4-Hydroxybenzoic acid |
6.130 |
C7H6O3 |
68.42±0.77 |
137.20 |
| Luteolin |
6.190 |
C15H10O6 |
733.81±1.50 |
285.20 |
| Curmin |
6.516 |
C21H19O6 |
108.71±1.15 |
367.00 |
| Each value is expressed as a mean ± standard deviation of three replicates (n = 3). RT: Retention time, MF: Molecular formula. |
3.5. NMR spectral and DSC thermal analyses of İsot seed oilTOP
Gas chromatography equipped with a flame ionization detector (GC-FID) is one of the best known methods for detecting the presence of fatty acids in the samples or the fatty acid composition of vegetable oils. NMR is currently being used along with mass spectrometry for detecting a wide range of fatty acids and phytochemicals including phenolic compounds. Compared to other analytical analyses, 1H-NMR is relatively fast and different chemical structures are accurately and simultaneously determined (Savorani et al., 2013). The 1H-NMR spectrum obtained in this study represented olefin protons at 5.28 ppm, bis-allylic protons at 2.71 ppm, allylic protons at 1.98 ppm and terminal methyl group protons at 0.83 ppm (Figure 1A). The unsaturated nature of PSO was also confirmed by NMR analysis. Linoleic acid was the most abundant fatty acid present in PSO and accounted for almost 74% of the total fatty acids found in PSO. Linoleic acid carbons, including the protons attached to olefinic carbons (–CH=CH–; C9,10,12,13), bis-allylic protons (=CH–CH2–CH=; C11), the allylic/α-C protons (=CH–CH2-alkyl; C: 2,8,14), and alkyl protons (–CH2/–CH3; C: 3,4,5,6,7,15,16,17,18) were clearly visible on the 1H-NMR spectrum (Figure 1A). The homonuclear 2D – 1H NMR COSY experiment contained apparent cross-peaks between the –CH= protons at 5.2 ppm and neighboring –CH2– groups at 2.01 ppm. The other cross-peak of the –CH= protons at 5.2 ppm was at 2.74 ppm and arose from the –CH2– groups between two double bonds (Figure 1B). This peak is generally found in the samples containing polyunsaturated fats. The DSC heating and cooling curves from -40 °C to 40 °C of PSO are given in Figure 2. The volume narrowing and exothermic effect resulting from crystallization started at -11.51 °C (Ton) and finished at -14.14 °C (Toff) with ΔH of 0.92 j/g. Ton, Toff and ΔH of the endothermic peak represented melting and were -15.84 °C, -17.38 °C and 0.64 j/g, respectively. One endothermic peak and 1 exothermic peak were detected. Previous thermal behavior evaluated in sunflower oil indicated the presence of three endothermic peaks and one exothermic peak (Calligaris et al., 2004). The differences in the number of the peaks could be due to the complexity of the Triacylglycerol (TAG) distribution (Tan and Che Man, 2002) because TAG could be present in different forms such as polymorphic and crystalline structures (Desmedt et al., 1990). In addition, TAG with high saturated fatty acids melt at higher temperatures compare to highly unsaturated ones (Calligaris et al., 2004). According to Myat et al. (2009), crystallization and melting temperatures directly depend on SFA/PUFA and SFA/MUFA ratios.
|
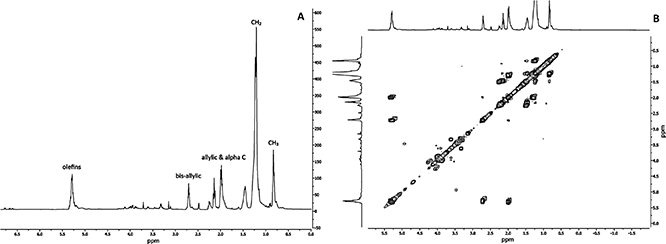 Figure 1. 400 Mhz -1H NMR spectrum for pepper seed oil. Each point corresponds to the average value of three replicates. The relevant NMR spectral features are defined as olefinic protons (-CH=CH-), the bis-allylic protons (=CH-CH2-CH=), the allylic/α-C protons (=CH-CH2-alkyl), the alkyl and terminal methyl protons (-CH2/-CH3; C) (A). Two-dimensional homonuclear NMR correlation spectroscopy (2D NMR COSY) of pepper seed oil (B). Figure 1. 400 Mhz -1H NMR spectrum for pepper seed oil. Each point corresponds to the average value of three replicates. The relevant NMR spectral features are defined as olefinic protons (-CH=CH-), the bis-allylic protons (=CH-CH2-CH=), the allylic/α-C protons (=CH-CH2-alkyl), the alkyl and terminal methyl protons (-CH2/-CH3; C) (A). Two-dimensional homonuclear NMR correlation spectroscopy (2D NMR COSY) of pepper seed oil (B).
|
|
|
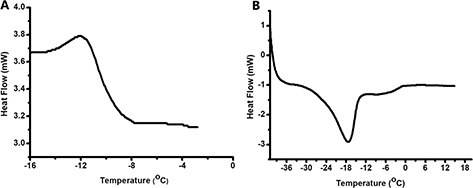 Figure 2. DSC thermogram for crystallization (A) and melting process (B). Each one of the two curves presented is representative of three different matching thermograms. Figure 2. DSC thermogram for crystallization (A) and melting process (B). Each one of the two curves presented is representative of three different matching thermograms.
|
|
3.6. Antimicrobial, anti-diabetic and antioxidant activities of İsot seed oilTOP
The antimicrobial properties of PSO were assessed against Gram-negative, Gram-positive bacteria, yeast and fungi, including
E. coli, S. aureus, C. albicans, A. brasiliensis. The Agar well diffusion assay was used to test the antimicrobial activity of PSO and the diameters of the inhibition zones obtained are shown in Table 5. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of PSO on pathogenic microorganisms were also determined. The aqueous extract of PSO showed bactericidal activity only against S. aureus, which is a Gram-Positive bacterium. There is no study in the literature describing the antimicrobial activity of PSO. However, some studies report the antimicrobial activity of aqueous extracts of olive oil. The antimicrobial activity of aqueous extracts of olive oil was tested against Salmonella enteritidis, Listeria monocytogenes, S. aureus, E. coli O157:H7, Yersinia sp. 5057655, and S. sonnei JCP, and showed bactericidal activity against the food borne pathogens tested (Medina et al., 2007). The aqueous extract of virgin olive oil exerted strong antimicrobial activity. Neither S. aureus nor Yersinia sp. was detected after 5 min of contact with the aqueous extract of virgin olive oil, which represents as 5 log reduction in the count of pathogens (Medina et al., 2007). In another study, extra virgin olive oil (EVOO) showed strong bactericidal activity, although refined oils were found to be ineffective. The percent inhibition results of the phenolic compounds in EVOO were found at up to 13.13% for E. coli O157:H7, up to 27.68% for L. monocytogenes and up to 11.64% for S. enteritidis (Karaosmanoğlu, 2010). There are few studies regarding the antimicrobial activity of edible oils when compared to essential oils. The essential oils obtained from various plant tissues including leaves, flowers, stems, fruits and seeds displayed antimicrobial activity against various bacterial strains (Semeniuc et al., 2017). Much of the emphasis was devoted to the antimicrobial activity of essential oils rather than edible ones. It was generally shown that plant extracts are usually more potent inhibitors of Gram-positive bacteria than Gram-negative bacteria (Lin et al., 1999). Similar findings were also presented in this study. The aqueous extract of PSO exerted antimicrobial activity against a Gram-positive bacterium while no inhibitory activity was observed against Gram-negative E. coli. We were not able to detect any inhibitory activity against mold (A. brasiliensis) or yeast (C. albicans) cultures either. The inhibitory effects of PSO extracts on α-amylase and α-glucosidase enzymes are shown in Table 5. The extracts were found incapable on preventing α-amylase activity but exhibited inhibitory activity against α-glucosidase with an IC50 value of 22.29±0.20 mg/mL. Acarbose was used as a positive control. The PSO extracts possessed the same inhibitory effect (p > 0.05) against α-glucosidase as determined for acarbose (20.28±0.91 mg/mL), indicating that it was capable inhibiting the active center (Rubilar et al., 2011). The enzyme activity of PSO could be explained by its bioactive compounds, including phenolic, carotenoid, capsaicinoid and tocopherol contents. Due to lack of studies on the enzymatic activities of oils, the results were compared to the results of other extracts, such as spinach (6.03 μg/mL) (Vyas, 2017). As expected, the plant extracts were more potent than the PSO aqueous extract in blocking the active center of the assayed enzymes. We tested the antioxidant activity of PSO by applying four different methods, including DPPH, ABTS, CUPRAC, and FRAP as a single method did not fully describe the antioxidant capacity of the samples (Table 5). DPPH-ABTS are radical scavenging and FRAP-CUPRAC are reducing power assays, and the results were expressed as μmol TEAC/g, as shown in Table 5. The radical scavenging ability of the assayed PSO was approximately 1.5 times higher in the DPPH assay (11.38±0.31 μmol TEAC/g) (p < 0.05) when compared to the ABTS assay (7.81 ± 0.01 μmol TEAC/g). The reducing power activities of PSO according to the CUPRAC and FRAP assays were also evaluated and varied significantly (p < 0.05), revealing 8.55 ± 0.30 and 3.89 ± 0.13 μmol TEAC/g, respectively.
Table 5. Antimicrobial, antioxidant and anti-diabetic activities of pepper seed oil
| Pepper Seed Oil |
| |
MIC (mg/mL) |
MBC (mg/mL) |
IZ (cm) |
Anti-diabetic activity (mg/mL) |
Antioxidant Activity(μmol TEAC/g) |
| E. coli (ATCC 8739) |
nd |
nd |
nd |
α-amylase nd |
DPPH 11.38±0.31* |
| S. aureus (ATCC 6538) |
50 |
100 |
1.3 |
α-glucosidase 22.29±0.20* |
ABTS 7.81±0.01 |
| C. albicans (ATCC 10231) |
nd |
nd |
nd |
|
CUPRAC 8.55±0.30 |
| A. brasiliensis (ATCC 16404) |
nd |
nd |
nd |
|
FRAP 3.89±0.13 |
| Each value is expressed as a mean ± standard deviation of three replicates (n = 3). Statistically significant differences among results in the same column at the confidence level of 95% for each parameter are marked with *. nd: not detected, MIC: Minimum inhibition concentration, MBC: Minimum bacterial concentration, IZ: Inhibition zone, DPPH: 2,2-Di (4-tert-octylphenyl)-1-picrylhydrazyl, ABTS: 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid, CUPRAC: Cupric reducing antioxidant capacity, FRAP: Ferric reducing antioxidant power, TEAC: Trolox equivalent antioxidant capacity. |
4. CONCLUSIONSTOP
Data on phytochemical content, phenolic composition, enzyme inhibitory and antimicrobial activity in PSO are scarce in the scientific literature. This study showed that the PSO obtained from İsot pepper seeds had considerable amounts of unsaturated fatty acids, capsaicin, carotenoid and tocopherol, which are considered beneficial for human health. The LC-ESI-MS/MS indicated a broad complexity in the PSO phenolic composition and allowed for the identification of 14 distinct compounds. Resveratrol, a potent antioxidant and health-promoting compound, represented at a high concentration in PSO. The inhibitory activity of PSO on the growth of pathogenic microorganisms, free radical scavenging activity, was thought to be related to its protective and antioxidant potential. Today, many people are not be reaching micronutrient intake requirements from the diet alone as they consume energy-rich, micronutrient-poor foods. Phytochemicals, vitamins, antioxidants, and minerals are needed for an adequately balanced diet. Integrating PSO to foods represents a healthy way to supply micronutrient requirements and improve the quality of life through its antioxidant, antimicrobial, and nutritional powers.
ACKNOWLEDGMENTSTOP
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
REFERENCESTOP
| ○ |
AOAC. 1984. Official Methods of Analysis, vol. 67, 14th ed. Association of Official Analytical Chemists, Arlington, VA. pp. 503–515. |
| ○ |
Apak R, Güçlü K, Özyürek M, Çelik SE. 2007. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 160, 413–419. https://doi.org/10.1007/s00604-007-0777-0 |
| ○ |
Arimboor R, Natarajan RB, Menon KR, Chandrasekhar LP, Moorkoth V. 2014. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—a review. J. Food Sci. Technol. 52, 1258–1271. https://doi.org/10.1007/s13197-014-1260-7 |
| ○ |
Asnin L, Park SW. 2015. Isolation and analysis of bioactive compounds in Capsicum peppers. Crit. Rev. Food Sci. Nutr. 55 (2), 254–289. https://doi.org/10.1080/10408398.2011.652316 |
| ○ |
Baenas N, Belović M, Ilic N, Moreno DA, García-Viguera C. 2019. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 274, 872–885. https://doi.org/10.1016/j.foodchem.2018.09.047 |
| ○ |
Baur JA, Sinclair DA. 2006. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506. https://doi.org/10.1038/nrd2060 |
| ○ |
Benzie IFF, Strain JJ. 1996. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Anal. Biochem. 239, 70–76. https://doi.org/10.1006/abio.1996.0292 |
| ○ |
Bosland PW, Votava EJ. 2000. Introduction. Peppers: Vegetable and spice capsicums. CABI. 1–12. https://doi.org/10.1079/9781845938253.0001 |
| ○ |
Calligaris S, Manzocco L, Conte LS, Nicoli MC. 2004. Application of a modified arrhenius equation for the evaluation of oxidation rate of sunflower oil at subzero temperatures. J. Food Sc. 69, 361–366. https://doi.org/10.1111/j.1365-2621.2004.tb09896.x |
| ○ |
Çam M, Hışıl Y, Durmaz G. 2009. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 112, 721–726. https://doi.org/10.1016/j.foodchem.2008.06.009 |
| ○ |
Chen L, Kang YH. 2013. In vitro inhibitory effect of oriental melon (Cucumis melo L. var. makuwa Makino) seed on key enzyme linked to type 2 diabetes: Assessment of anti-diabetic potential of functional food. J. Funct Foods 5 (2), 981–986. https://doi.org/10.1016/j.jff.2013.01.008
|
| ○ |
Chougui N, Tamendjari A, Hamidj W, Hallal S, Barras A, Richard T, Larbat R. 2013. Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 139, 796–803. https://doi.org/10.1016/j.foodchem.2013.01.054
|
| ○ |
Collins MD, Wasmund LM, Bosland PW. 1995. Improved method for quantifying Capsaicinoids in capsicum using high-performance liquid chromatography. Hort. Sci. 30, 137–139. https://doi.org/10.21273/HORTSCI.30.1.137 |
| ○ |
Desmedt A, Culot C, Deroanne C, Durant F, Gibon V. 1990. Influence of cis and trans double bonds on the thermal and structural properties of monoacid triglycerides. J. Am. Oil Chem. Soc. 67, 653–660. https://doi.org/10.1007/BF02540417 |
| ○ |
Di Sotto A, Vecchiato M, Abete L, Toniolo C, Giusti AM, Mannina L, Locatelli M, Nicoletti M, Di Giacomo S. 2018. Capsicum annuum L. var. Cornetto di Pontecorvo PDO: Polyphenolic profile and in vitro biological activities. J. Funct. Foods. 40, 679–691. https://doi.org/10.1016/j.jff.2017.11.041 |
| ○ |
El-Seedi HR, Taher EA, Sheikh BY, Anjum S, Saeed A, AlAjmi MF, Göransson U. 2018. Hydroxycinnamic Acids: Natural sources, biosynthesis, possible biological activities, and roles in Islamic Medicine. Stud. Nat. Prod. Chem. 55, 269–292. https://doi.org/10.1016/B978-0-444-64068-0.00008-5 |
| ○ |
Figueiredo NR, Meena M, Soni A. 2013. Capsaicin: a review. J. Dentofacial. Sci. Available from http://journalofdentofacialsciences.com (Accessed 27.11.13). |
| ○ |
Fromm M, Bayha S, Kammerer DR, Carle R. 2012. Identification and quantitation of carotenoids and tocopherols in seed oils recovered from different rosaceae species. J. Agric. Food Chem. 60, 10733–10742. https://doi.org/10.1021/jf3028446 |
| ○ |
Hayman M, Kam PCA. 2008. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care. 19, 338–343.
|
| ○ |
Jarret RL, Levy IJ, Potter TL, Cermak SC. 2013. Seed oil and fatty acid composition in Capsicum spp. J. Food Compos. Anal. 30, 102–108. https://doi.org/10.1016/j.jfca.2013.02.005 |
| ○ |
Jayaprakasha GK, Bae H, Crosby K, Jifon JL, Patil BS. 2012. Bioactive compounds in peppers and their antioxidant potential. Hispanic Foods: Chemistry and Bioactive Compounds 43–56. https://doi.org/10.1021/bk-2012-1109.ch004 |
| ○ |
Karaosmanoglu H, Soyer F, Ozen B, Tokatli F. 2010. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J. Agric. Food Chem. 58 (14), 8238–8245. https://doi.org/10.1021/jf1012105 |
| ○ |
Lin J, Opoku AR, Geheeb-Keller M, Hutchings AD, Terblanche SE, K. Jäger A, Van Staden J. 1999. Preliminary screening of some traditional zulu medicinal plants for anti-inflammatory and anti-microbial activities. J. Ethnopharmacol. 68, 267–274. https://doi.org/10.1016/S0378-8741(99)00130-0
|
| ○ |
Liu Y, Huang J, Zheng X, Yang X, Ding Y, Fang T, Huang XF. 2017. Luteolin, a natural flavonoid, inhibits methylglyoxal induced apoptosis via the mTOR/4E-BP1 signaling pathway. Sci. Rep. 7, 7877. https://doi.org/10.1038/s41598-017-08204-6 |
| ○ |
Materska M, Perucka I. 2005. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 53, 1750–1756. https://doi.org/10.1021/jf035331k |
| ○ |
McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. 2005. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 53, 2760–2766. https://doi.org/10.1021/jf0489926 |
| ○ |
Medina E, Romero C, Brenes M, De Castro A. 2007. Antimicrobial activity of olive oil, vinegar, and various beverages against Foodborne Pathogens. J. Food Prot. 70, 1194–1199. https://doi.org/10.4315/0362-028X-70.5.1194 |
| ○ |
Myat MW, Abdulkarim SM, Ghazali HM, Roselina K. 2009. Physicochemical and sensory characteristics of palm olein and peanut oil blends. J. Food Agric. Environ. 7, 175–181
|
| ○ |
Othman ZAA, Ahmed YBH, Habila MA, Ghafar AA. 2011. Determination of capsaicin and dihydrocapsaicin in capsicum fruit samples using high performance liquid chromatography. Molecules 16, 8919–8929. https://doi.org/10.3390/molecules16108919 |
| ○ |
Pandey KB, Rizvi SI. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2, 270–278. https://doi.org/10.4161/oxim.2.5.9498 |
| ○ |
Pinheiro-Sant’Ana HM, Guinazi M, Oliveira, da Silva Oliveira D, Della Lucia CM, Reis BdeL, Brandão SCC. 2011. Method for simultaneous analysis of eight vitamin E isomers in various foods by high performance liquid chromatography and fluorescence detection. J. Chromatogr. 1218, 8496–8502. https://doi.org/10.1016/j.chroma.2011.09.067 |
| ○ |
Rubilar M, Jara C, Poo Y, Acevedo F, Gutierrez C, Sineiro J, Shene C. 2011. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 59, 1630–1637. https://doi.org/10.1021/jf103461k |
| ○ |
Sandoval-Castro CJ, Valdez-Morales M, Oomah BD, Gutiérrez-Dorado R, Medina-Godoy S, Espinosa-Alonso LG. 2017. Bioactive compounds and antioxidant activity in scalded Jalapeño pepper industrial byproduct (Capsicum annuum). J. Food Sci. Technol. 54, 1999–2010. https://doi.org/10.1007/s13197-017-2636-2
|
| ○ |
Savorani F, Rasmussen MA, Mikkelsen MS, Engelsen SB. 2013. A primer to nutritional metabolomics by NMR spectroscopy and chemometrics. Food Res. Int. 54 (1), 1131–1145. https://doi.org/10.1016/j.foodres.2012.12.025 |
| ○ |
Semeniuc CA, Pop CR, Rotar AM. 2017. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 25, 403–408. https://doi.org/10.1016/j.jfda.2016.06.002 |
| ○ |
Shahidi F, Ambigaipalan P. 2018. Omega-3 Polyunsaturated Fatty Acids and Their health benefits. Annu. Rev. Food Sci. Technol. 9, 345–381. https://doi.org/10.1146/annurev-food-111317-095850
|
| ○ |
Tan CP, Che Man YB. 2002. Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil:
Effects of scanning rate variation. Food Chem. 76, 89–102. https://doi.org/10.1016/S0308-8146(01)00241-2
|
| ○ |
Vyas M. 2017. Nutritional profile of spinach and its antioxidant and antidiabetic evaluation. Int. J. Green Pharm. 11, 192–197
|
| ○ |
Walsh BM, Hoot SB. 2001. Phylogenetic relationships of Capsicum (Solanaceae) using DNA sequences from two Noncoding Regions: The chloroplast atpB-rbcL spacer region and nuclear waxy introns. Int. J. Plant Sci. 162, 1409–1418. https://doi.org/10.1086/323273 |
| ○ |
Yılmaz E, Sevgi Arsunar E, Aydeniz B, Güneşer O. 2015. Cold pressed capia pepperseed (Capsicum Annuum L.) oils: Composition, aroma, and sensory properties. Eur. J. Lipid Sci. Technol. 117, 1016–1026. https://doi.org/10.1002/ejlt.201400276 |
| ○ |
Zhishen J, Mengcheng T, Jianming W. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559. https://doi.org/10.1016/S0308-8146(98)00102-2 |
Figure 1. 400 Mhz -1H NMR spectrum for pepper seed oil. Each point corresponds to the average value of three replicates. The relevant NMR spectral features are defined as olefinic protons (-CH=CH-), the bis-allylic protons (=CH-CH2-CH=), the allylic/α-C protons (=CH-CH2-alkyl), the alkyl and terminal methyl protons (-CH2/-CH3; C) (A). Two-dimensional homonuclear NMR correlation spectroscopy (2D NMR COSY) of pepper seed oil (B).